Influenza virus activates inflammasomes via its intracellular M2 ion channel
- PMID: 20383149
- PMCID: PMC2857582
- DOI: 10.1038/ni.1861
VSports注册入口 - Influenza virus activates inflammasomes via its intracellular M2 ion channel
Abstract
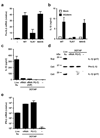
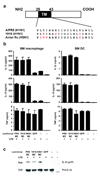
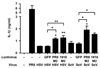
Influenza virus, a negative-stranded RNA virus that causes severe illness in humans and animals, stimulates the inflammasome through the Nod-like receptor NLRP3. However, the mechanism by which influenza virus activates the NLRP3 inflammasome is unknown. Here we show that the influenza virus M2 protein, a proton-selective ion channel important in viral pathogenesis, stimulates the NLRP3 inflammasome pathway. M2 channel activity was required for the activation of inflammasomes by influenza and was sufficient to activate inflammasomes in primed macrophages and dendritic cells VSports手机版. M2-induced activation of inflammasomes required its localization to the Golgi apparatus and was dependent on the pH gradient. Our results show a mechanism by which influenza virus infection activates inflammasomes and identify the sensing of disturbances in intracellular ionic concentrations as a previously unknown pathogen-recognition pathway. .
Figures (VSports手机版)





"VSports最新版本" References
-
- Kanneganti TD, et al. Critical role for Cryopyrin/Nalp3 in activation of caspase-1 in response to viral infection and double-stranded RNA. J Biol Chem. 2006;281:36560–36568. - PubMed
-
- Thomas PG, et al. The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity. 2009;30:566–575. - PMC (V体育官网) - PubMed
-
- Martinon F, Tschopp J. Inflammatory caspases: linking an intracellular innate immune system to autoinflammatory diseases. Cell. 2004;117:561. - PubMed
Publication types
MeSH terms
- "VSports最新版本" Actions
- Actions (V体育官网入口)
- "V体育官网入口" Actions
- Actions (V体育安卓版)
- VSports - Actions
- V体育官网入口 - Actions
- "V体育官网" Actions
- Actions (V体育ios版)
- V体育2025版 - Actions
- VSports注册入口 - Actions
- Actions (V体育2025版)
- "VSports" Actions
- "V体育平台登录" Actions
- Actions (VSports)
- "VSports最新版本" Actions
- Actions (V体育官网入口)
- Actions (VSports在线直播)
- "V体育ios版" Actions
- VSports注册入口 - Actions
- "VSports手机版" Actions
- Actions (VSports手机版)
- "V体育安卓版" Actions
- Actions (VSports在线直播)
- Actions (V体育安卓版)
- V体育官网入口 - Actions
- Actions (V体育ios版)
- "V体育平台登录" Actions
- "V体育平台登录" Actions
- VSports - Actions
- Actions (VSports注册入口)
Substances
- "VSports app下载" Actions
- VSports app下载 - Actions
- VSports在线直播 - Actions
- "VSports app下载" Actions
- Actions (VSports手机版)
- Actions (V体育官网入口)
- "V体育平台登录" Actions
- "V体育2025版" Actions
VSports在线直播 - Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

