Oxygen activation at mononuclear nonheme iron centers: a superoxo perspective
- PMID: 20380464
- PMCID: PMC2864595
- DOI: 10.1021/ic901891n
Oxygen activation at mononuclear nonheme iron centers: a superoxo perspective (VSports)
Abstract
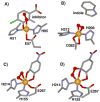
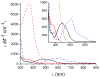
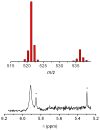
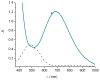
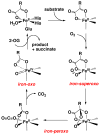
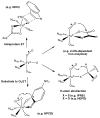
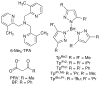
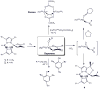
Dioxygen (O(2)) activation by iron enzymes is responsible for many metabolically important transformations in biology. Often a high-valent iron oxo oxidant is proposed to form upon O(2) activation at a mononuclear nonheme iron center, presumably via intervening iron superoxo and iron peroxo species VSports手机版. While iron(IV) oxo intermediates have been trapped and characterized in enzymes and models, less is known of the putative iron(III) superoxo species. Utilizing a synthetic model for the 2-oxoglutarate-dependent monoiron enzymes, [(Tp(iPr2))Fe(II)(O(2)CC(O)CH(3))], we have obtained indirect evidence for the formation of the putative iron(III) superoxo species, which can undergo one-electron reduction, hydrogen-atom transfer, or conversion to an iron(IV) oxo species, depending on the reaction conditions. These results demonstrate the various roles that the iron(III) superoxo species can play in the course of O(2) activation at a nonheme iron center. .
Figures

References (VSports app下载)
-
- Solomon EI, Brunold TC, Davis MI, Kemsley JN, Lee SK, Lehnert N, Neese F, Skulan AJ, Yang YS, Zhou J. Chem Rev. 2000;100:235–349. - PubMed (V体育ios版)
-
- Costas M, Mehn MP, Jensen MP, Que L., Jr Chem Rev. 2004;104:939–986. - PubMed
-
- Hausinger RP. Crit Rev Biochem Mol Biol. 2004;39:21–68. - PubMed
-
- Loenarz C, Schofield CJ. Nature Chem Biol. 2008;4:152–156. - PubMed
Publication types
- Actions (VSports app下载)
MeSH terms
- VSports手机版 - Actions
- "V体育安卓版" Actions
- Actions (VSports)
- Actions (VSports app下载)
- Actions (V体育官网入口)
Substances
- Actions (V体育2025版)
- "V体育官网" Actions
- Actions (V体育ios版)
Grants and funding (VSports最新版本)
LinkOut - more resources
VSports手机版 - Full Text Sources

