Using Epstein-Barr viral load assays to diagnose, monitor, and prevent posttransplant lymphoproliferative disorder
- PMID: 20375356
- PMCID: PMC2863367
- DOI: VSports在线直播 - 10.1128/CMR.00006-09
Using Epstein-Barr viral load assays to diagnose, monitor, and prevent posttransplant lymphoproliferative disorder
Abstract
Epstein-Barr virus (EBV) DNA measurement is being incorporated into routine medical practice to help diagnose, monitor, and predict posttransplant lymphoproliferative disorder (PTLD) in immunocompromised graft recipients. PTLD is an aggressive neoplasm that almost always harbors EBV DNA within the neoplastic lymphocytes, and it is often fatal if not recognized and treated promptly. Validated protocols, commercial reagents, and automated instruments facilitate implementation of EBV load assays by real-time PCR. When applied to either whole blood or plasma, EBV DNA levels reflect clinical status with respect to EBV-related neoplasia. While many healthy transplant recipients have low viral loads, high EBV loads are strongly associated with current or impending PTLD. Complementary laboratory assays as well as histopathologic examination of lesional tissue help in interpreting modest elevations in viral load. Circulating EBV levels in serial samples reflect changes in tumor burden and represent an effective, noninvasive tool for monitoring the efficacy of therapy VSports手机版. In high-risk patients, serial testing permits early clinical intervention to prevent progression toward frank PTLD. Restoring T cell immunity against EBV is a major strategy for overcoming PTLD, and novel EBV-directed therapies are being explored to thwart virus-driven neoplasia. .
Figures
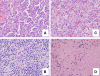
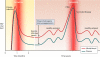
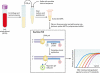

References
-
- Reference deleted.
-
- Aalto, S. M., E. Juvonen, J. Tarkkanen, L. Volin, H. Haario, T. Ruutu, and K. Hedman. 2007. Epstein-Barr viral load and disease prediction in a large cohort of allogeneic stem cell transplant recipients. Clin. Infect. Dis. 45:1305-1309. - PubMed
-
- Ahmad, I., N. V. Cau, J. Kwan, Y. Maaroufi, N. Meuleman, M. Aoun, P. Lewalle, P. Martiat, F. Crokaert, and D. Bron. 2009. Preemptive management of Epstein-Barr virus reactivation after hematopoietic stem-cell transplantation. Transplantation 87:1240-1245. - PubMed
-
- Ahsanuddin, A. N., M. C. Standish, A. M. Caliendo, C. E. Hill, and F. S. Nolte. 2008. Validation of an Epstein-Barr viral load assay using the QIAGEN Artus EBV TM PCR analyte-specific reagent. Am. J. Clin. Pathol. 130:865-869. - PubMed (V体育ios版)
-
- Ahya, V. N., L. P. Douglas, C. Andreadis, S. Arnoldi, J. Svoboda, R. M. Kotloff, D. Hadjiliadis, J. S. Sager, Y. J. Woo, A. Pochettino, S. J. Schuster, E. A. Stadtmauer, and D. E. Tsai. 2007. Association between elevated whole blood Epstein-Barr virus (EBV)-encoded RNA EBV polymerase chain reaction and reduced incidence of acute lung allograft rejection. J. Heart Lung Transplant. 26:839-844. - "VSports最新版本" PubMed
Publication types
- "VSports最新版本" Actions
"VSports手机版" MeSH terms
- VSports app下载 - Actions
- Actions (VSports在线直播)
- Actions (VSports最新版本)
- "V体育2025版" Actions
- Actions (V体育官网入口)
- VSports在线直播 - Actions
Substances
- "VSports" Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

