Caveolin-1-dependent occludin endocytosis is required for TNF-induced tight junction regulation in vivo
- PMID: 20351069
- PMCID: PMC2854371
- DOI: 10.1083/jcb.200902153
Caveolin-1-dependent occludin endocytosis is required for TNF-induced tight junction regulation in vivo
Abstract
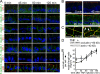
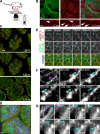
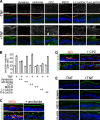
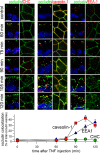
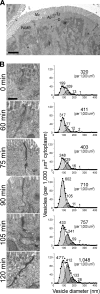
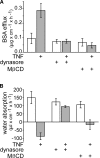
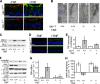
Epithelial paracellular barrier function, determined primarily by tight junction permeability, is frequently disrupted in disease. In the intestine, barrier loss can be mediated by tumor necrosis factor (alpha) (TNF) signaling and epithelial myosin light chain kinase (MLCK) activation. However, TNF induces only limited alteration of tight junction morphology, and the events that couple structural reorganization to barrier regulation have not been defined. We have used in vivo imaging and transgenic mice expressing fluorescent-tagged occludin and ZO-1 fusion proteins to link occludin endocytosis to TNF-induced tight junction regulation VSports手机版. This endocytosis requires caveolin-1 and is essential for structural and functional tight junction regulation. These data demonstrate that MLCK activation triggers caveolin-1-dependent endocytosis of occludin to effect structural and functional tight junction regulation. .
Figures


References
-
- Balda M.S., Whitney J.A., Flores C., González S., Cereijido M., Matter K. 1996. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J. Cell Biol. 134:1031–1049 10.1083/jcb.134.4.1031 - DOI - PMC - PubMed
-
- Berglund J.J., Riegler M., Zolotarevsky Y., Wenzl E., Turner J.R. 2001. Regulation of human jejunal transmucosal resistance and MLC phosphorylation by Na(+)-glucose cotransport. Am. J. Physiol. Gastrointest. Liver Physiol. 281:G1487–G1493 - PubMed (V体育2025版)
Publication types
MeSH terms
- Actions (VSports在线直播)
- Actions (V体育2025版)
- V体育ios版 - Actions
- V体育平台登录 - Actions
- Actions (V体育官网入口)
- "V体育ios版" Actions
- Actions (V体育官网入口)
Substances
- Actions (VSports)
- V体育2025版 - Actions
- "VSports最新版本" Actions
- "V体育安卓版" Actions
- Actions (VSports最新版本)
Grants and funding
- P01 DK067887/DK/NIDDK NIH HHS/United States (VSports在线直播)
- P30CA14599/CA/NCI NIH HHS/United States
- UL1 RR024999/RR/NCRR NIH HHS/United States
- P30 CA014599/CA/NCI NIH HHS/United States
- R21 DK074976/DK/NIDDK NIH HHS/United States
- "VSports在线直播" T32 HL007237/HL/NHLBI NIH HHS/United States
- R01 DK061931/DK/NIDDK NIH HHS/United States
- R01 DK068271/DK/NIDDK NIH HHS/United States
- R01DK61931/DK/NIDDK NIH HHS/United States
- UL1RR024999/RR/NCRR NIH HHS/United States
- T32HL007237/HL/NHLBI NIH HHS/United States
- P01DK67887/DK/NIDDK NIH HHS/United States
- R01DK68271/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
V体育官网 - Other Literature Sources
Molecular Biology Databases

