Absence of glutathione peroxidase 4 affects tumor angiogenesis through increased 12/15-lipoxygenase activity
- PMID: 20234819
- PMCID: "V体育官网" PMC2838443
- DOI: "V体育官网入口" 10.1593/neo.91782
Absence of glutathione peroxidase 4 affects tumor angiogenesis through increased 12/15-lipoxygenase activity
Abstract
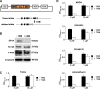
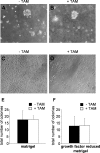
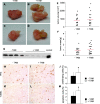
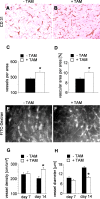
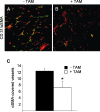
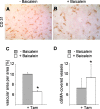
The selenoenzyme glutathione peroxidase 4 (GPx4) has been described to control specific cyclooxygenases (COXs) and lipoxygenases (LOXs) that exert substantiated functions in tumor growth and angiogenesis. Therefore, we hypothesized a putative regulatory role of GPx4 during tumor progression and created transformed murine embryonic fibroblasts with inducible disruption of GPx4 VSports手机版. GPx4 inactivation caused rapid cell death in vitro, which could be prevented either by lipophilic antioxidants or by 12/15-LOX-specific inhibitors, but not by inhibitors targeting other LOX isoforms or COX. Surprisingly, transformed GPx4(+/-) cells did not die when grown in Matrigel but gave rise to tumor spheroids. Subcutaneous implantation of tumor cells into mice resulted in knockout tumors that were indistinguishable in volume and mass in comparison to wild-type tumors. However, further analysis revealed a strong vascular phenotype. We observed an increase in microvessel density as well as a reduction in the number of large diameter vessels covered by smooth muscle cells. This phenotype could be linked to increased 12/15-LOX activity that was accompanied by an up-regulation of basic fibroblast growth factor and down-regulation of vascular endothelial growth factor A protein expression. Indeed, pharmacological inhibition of 12/15-LOX successfully reversed the tumor phenotype and led to "normalized" vessel morphology. Thus, we conclude that GPx4, through controlling 12/15-LOX activity, is an important regulator of tumor angiogenesis as well as vessel maturation. .
Figures
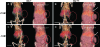
References
-
- Ursini F, Maiorino M, Valente M, Ferri L, Gregolin C. Purification from pig liver of a protein which protects liposomes and biomembranes from peroxidative degradation and exhibits glutathione peroxidase activity on phosphatidylcholine hydroperoxides. Biochim Biophys Acta. 1982;710:197–211. - PubMed
-
- Imai H, Narashima K, Arai M, Sakamoto H, Chiba N, Nakagawa Y. Suppression of leukotriene formation in RBL-2H3 cells that overexpressed phospholipid hydroperoxide glutathione peroxidase. J Biol Chem. 1998;273:1990–1997. - VSports - PubMed
-
- Hemler ME, Crawford CG, Lands WE. Lipoxygenation activity of purified prostaglandin-forming cyclooxygenase. Biochemistry. 1978;17:1772–1779. - PubMed
-
- Lands WE. Interactions of lipid hydroperoxides with eicosanoid biosynthesis. J Free Radic Biol Med. 1985;1:97–101. - "V体育平台登录" PubMed
-
- Fürstenberger G, Krieg P, Müller-Decker K, Habenicht AJR. What are cyclooxygenases and lipoxygenases doing in the driver's seat of carcinogenesis? Int J Cancer. 2006;119:2247–2254. - PubMed
Publication types (VSports在线直播)
MeSH terms
- Actions (V体育官网入口)
- "V体育ios版" Actions
- "VSports最新版本" Actions
- "VSports手机版" Actions
- VSports注册入口 - Actions
- V体育ios版 - Actions
- Actions (V体育2025版)
- "V体育官网" Actions
- V体育ios版 - Actions
- "VSports手机版" Actions
- VSports app下载 - Actions
- "VSports注册入口" Actions
- V体育平台登录 - Actions
- Actions (VSports)
- "V体育安卓版" Actions
- "V体育平台登录" Actions
- "V体育平台登录" Actions
- "VSports" Actions
- "VSports手机版" Actions
- VSports手机版 - Actions
- "V体育ios版" Actions
- "V体育ios版" Actions
- V体育官网入口 - Actions
- VSports app下载 - Actions
Substances
- VSports最新版本 - Actions
- "V体育官网" Actions
- "VSports手机版" Actions
- V体育平台登录 - Actions
- "V体育2025版" Actions
- "V体育ios版" Actions
- VSports注册入口 - Actions
- "V体育官网" Actions
- V体育ios版 - Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
