Combination of IL-21 and IL-15 enhances tumour-specific cytotoxicity and cytokine production of TCR-transduced primary T cells (V体育官网入口)
- PMID: 20101507
- PMCID: "V体育2025版" PMC11030877
- DOI: 10.1007/s00262-010-0818-0
Combination of IL-21 and IL-15 enhances tumour-specific cytotoxicity and cytokine production of TCR-transduced primary T cells
Abstract
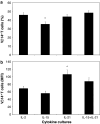
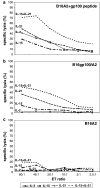
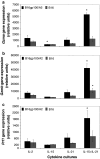
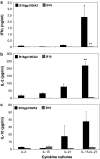
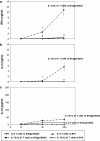
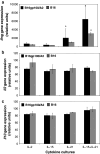
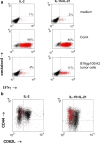
IL-21, and to a lesser extent IL-15, inhibits differentiation of antigen-primed CD8 T cells and promotes their homeostasis and anti-tumour activity. Here, we investigated molecular mechanisms behind tumour-specific responses of primary murine T lymphocytes engineered to express a TCR directed against human gp100/HLA-A2 following short-term exposure to IL-15 and/or IL-21. We demonstrated that IL-15 + IL-21, and to a lesser extent IL-21, enhanced antigen-specific T-cell cytotoxicity, which was related to enhanced expression of granzymes A and B, and perforin 1. Furthermore, IL-15 + IL-21 synergistically enhanced release levels and kinetics of T-cell IFNgamma and IL-2, but not IL-10. Enhanced secretion of IFNgamma was accompanied by increased gene expression and cytosolic protein content, and was restricted to effector memory T cells. To summarize, we show that IL-15 + IL-21 improves antigen-specific responses of TCR-transduced effector T cells at multiple levels, which provides a rationale to treat T cells with a combination of these cytokines prior to their use in adoptive TCR gene therapy VSports手机版. .
"V体育2025版" Figures
References
-
- Clay TM, Custer MC, Sachs J, Hwu P, Rosenberg SA, Nishimura MI. Efficient transfer of a tumor antigen-reactive TCR to human peripheral blood lymphocytes confers anti-tumor reactivity. J Immunol. 1999;163:507–513. - PubMed
-
- Schaft N, Willemsen RA, de Vries J, Lankiewicz B, Essers BW, Gratama JW, Figdor CG, Bolhuis RL, Debets R, Adema GJ. Peptide fine specificity of anti-glycoprotein 100 CTL is preserved following transfer of engineered TCR alpha beta genes into primary human T lymphocytes. J Immunol. 2003;170:2186–2194. - PubMed
"VSports最新版本" Publication types
- Actions (VSports)
MeSH terms
- "VSports在线直播" Actions
- "VSports在线直播" Actions
- V体育官网入口 - Actions
- "V体育ios版" Actions
- "V体育官网入口" Actions
- Actions (VSports最新版本)
- "VSports最新版本" Actions
- "V体育ios版" Actions
- Actions (VSports在线直播)
- "VSports" Actions
- V体育安卓版 - Actions
- "V体育安卓版" Actions
- "V体育安卓版" Actions
- "VSports手机版" Actions
- "V体育官网" Actions
- "VSports最新版本" Actions
- V体育2025版 - Actions
- V体育平台登录 - Actions
- Actions (V体育安卓版)
- Actions (VSports手机版)
"VSports app下载" Substances
- VSports手机版 - Actions
- "V体育安卓版" Actions
- "VSports app下载" Actions
- Actions (VSports最新版本)
- Actions (V体育安卓版)
- "V体育官网" Actions
- VSports最新版本 - Actions
- "V体育官网" Actions
- VSports app下载 - Actions
- VSports - Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
"VSports" Medical
Research Materials

