Therapy of relapsed leukemia after allogeneic hematopoietic cell transplantation with T cells specific for minor histocompatibility antigens
- PMID: 20071660
- PMCID: PMC2869557
- DOI: 10.1182/blood-2009-10-248997
Therapy of relapsed leukemia after allogeneic hematopoietic cell transplantation with T cells specific for minor histocompatibility antigens
"VSports在线直播" Abstract
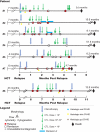
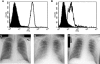
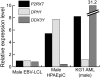
The adoptive transfer of donor T cells that recognize recipient minor histocompatibility antigens (mHAgs) is a potential strategy for preventing or treating leukemic relapse after allogeneic hematopoietic cell transplantation (HCT). A total of 7 patients with recurrent leukemia after major histocompatibility complex (MHC)-matched allogeneic HCT were treated with infusions of donor-derived, ex vivo-expanded CD8(+) cytotoxic T lymphocyte (CTL) clones specific for tissue-restricted recipient mHAgs. The safety of T-cell therapy, in vivo persistence of transferred CTLs, and disease response were assessed. Molecular characterization of the mHAgs recognized by CTL clones administered to 3 patients was performed to provide insight into the antileukemic activity and safety of T-cell therapy. Pulmonary toxicity of CTL infusion was seen in 3 patients, was severe in 1 patient, and correlated with the level of expression of the mHAg-encoding genes in lung tissue. Adoptively transferred CTLs persisted in the blood up to 21 days after infusion, and 5 patients achieved complete but transient remissions after therapy VSports手机版. The results of these studies illustrate the potential to selectively enhance graft-versus-leukemia activity by the adoptive transfer of mHAg-specific T-cell clones and the challenges for the broad application of this approach in allogeneic HCT. This study has been registered at http://clinicaltrials. gov as NCT00107354. .
"V体育安卓版" Figures
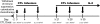



Comment in
-
V体育官网 - Combating cancer with allogeneic T cells.Blood. 2010 May 13;115(19):3856-7. doi: 10.1182/blood-2010-02-267328. Blood. 2010. PMID: 20466866 No abstract available.
References (VSports在线直播)
-
- Thomas ED. Bone marrow transplantation: a review. Semin Hematol. 1999;36(4) suppl 7:95–103. - VSports注册入口 - PubMed
-
- Horowitz MM, Gale RP, Sondel PM, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75(3):555–562. - PubMed
-
- Randolph SS, Gooley TA, Warren EH, Appelbaum FR, Riddell SR. Female donors contribute to a selective graft-versus-leukemia effect in male recipients of HLA-matched, related hematopoietic stem cell transplants. Blood. 2004;103(1):347–352. - "V体育官网" PubMed
-
- Collins RH, Jr, Shpilberg O, Drobyski WR, et al. Donor leukocyte infusions in 140 patients with relapsed malignancy after allogeneic bone marrow transplantation. J Clin Oncol. 1997;15(2):433–444. - PubMed
-
- Kolb HJ, Schattenberg A, Goldman JM, et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood. 1995;86(5):2041–2050. - V体育官网入口 - PubMed
Publication types
- Actions (VSports在线直播)
MeSH terms
- VSports在线直播 - Actions
- Actions (VSports app下载)
- VSports注册入口 - Actions
- "V体育平台登录" Actions
- VSports最新版本 - Actions
- Actions (V体育平台登录)
- Actions (V体育2025版)
- V体育平台登录 - Actions
- VSports在线直播 - Actions
- VSports app下载 - Actions
- "V体育官网入口" Actions
- "V体育官网入口" Actions
- Actions (VSports)
Substances
- V体育2025版 - Actions
Associated data
Grants and funding
VSports在线直播 - LinkOut - more resources
Full Text Sources
Other Literature Sources
V体育2025版 - Medical
Research Materials (VSports注册入口)

