The indispensable N-terminal half of eIF3j/HCR1 cooperates with its structurally conserved binding partner eIF3b/PRT1-RRM and with eIF1A in stringent AUG selection (V体育平台登录)
- PMID: 20060839
- PMCID: V体育平台登录 - PMC2824034
- DOI: "V体育ios版" 10.1016/j.jmb.2009.12.047
The indispensable N-terminal half of eIF3j/HCR1 cooperates with its structurally conserved binding partner eIF3b/PRT1-RRM and with eIF1A in stringent AUG selection
"VSports手机版" Abstract
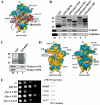
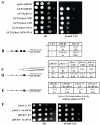
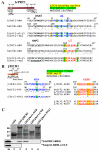
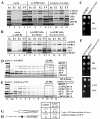
Despite recent progress in our understanding of the numerous functions of individual subunits of eukaryotic translation initiation factor (eIF) 3, little is known on the molecular level. Using NMR spectroscopy, we determined the first solution structure of an interaction between eIF3 subunits. We revealed that a conserved tryptophan residue in the human eIF3j N-terminal acidic motif (NTA) is held in the helix alpha1 and loop 5 hydrophobic pocket of the human eIF3b RNA recognition motif (RRM). Mutating the corresponding "pocket" residues in its yeast orthologue reduces cellular growth rate, eliminates eIF3j/HCR1 association with eIF3b/PRT1 in vitro and in vivo, affects 40S occupancy of eIF3, and produces a leaky scanning defect indicative of a deregulation of the AUG selection process VSports手机版. Unexpectedly, we found that the N-terminal half of eIF3j/HCR1 containing the NTA is indispensable and sufficient for wild-type growth of yeast cells. Furthermore, we demonstrate that deletion of either j/HCR1 or its N-terminal half only, or mutation of the key tryptophan residues results in the severe leaky scanning phenotype partially suppressible by overexpressed eIF1A, which is thought to stabilize properly formed preinitiation complexes at the correct start codon. These findings indicate that eIF3j/HCR1 remains associated with the scanning preinitiation complexes and does not dissociate from the small ribosomal subunit upon mRNA recruitment, as previously believed. Finally, we provide further support for earlier mapping of the ribosomal binding site for human eIF3j by identifying specific interactions of eIF3j/HCR1 with small ribosomal proteins RPS2 and RPS23 located in the vicinity of the mRNA entry channel. Taken together, we propose that eIF3j/HCR1 closely cooperates with the eIF3b/PRT1 RRM and eIF1A on the ribosome to ensure proper formation of the scanning-arrested conformation required for stringent AUG recognition. .
(c) 2009 Elsevier Inc V体育安卓版. All rights reserved. .
Figures




References
-
- Hinnebusch AG, Dever TE, Asano KA. Mechanism of translation initiation in the yeast Saccharomyces cerevisiae. In: Sonenberg N, Mathews M, Hershey JWB, editors. Translational Control in biology and medicine. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2007. pp. 225–268.
-
- Pestova TV, Lorsch JR, Hellen CUT. The mechanism of translation initiation in eukaryotes. In: Sonenberg N, Mathews M, Hershey JWB, editors. Translational Control in biology and medicine. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2007. pp. 87–128.
-
- Passmore LA, Schmeing TM, Maag D, Applefield DJ, Acker MG, Algire MA, Lorsch JR, Ramakrishnan V. The eukaryotic translation initiation factors eIF1 and eIF1A induce an open conformation of the 40S ribosome. Mol Cell. 2007;26:41–50. - PubMed
-
- Mitchell SF, Lorsch JR. Should I stay or should i go? Eukaryotic translation initiation factors 1 and 1a control start codon recognition. J Biol Chem. 2008 in press. - VSports最新版本 - PMC - PubMed
"V体育官网入口" Publication types
- VSports app下载 - Actions
MeSH terms
- "VSports app下载" Actions
- V体育官网 - Actions
- "V体育2025版" Actions
- "VSports在线直播" Actions
- Actions (VSports app下载)
- Actions (VSports注册入口)
- "VSports" Actions
- Actions (VSports)
- Actions (V体育ios版)
- "V体育官网" Actions
- "V体育官网入口" Actions
- "VSports手机版" Actions
- Actions (V体育ios版)
- "VSports注册入口" Actions
- VSports在线直播 - Actions
- VSports最新版本 - Actions
- "V体育官网" Actions
Substances
- V体育官网入口 - Actions
- V体育2025版 - Actions
- "VSports app下载" Actions
- V体育2025版 - Actions
- VSports app下载 - Actions
Associated data
- "V体育2025版" Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
"V体育ios版" Miscellaneous

