Early skin toxicity as a predictive factor for tumor control in hepatocellular carcinoma patients treated with sorafenib
- PMID: 20051477
- PMCID: PMC3227883
- DOI: 10.1634/theoncologist.2009-0143
Early skin toxicity as a predictive factor for tumor control in hepatocellular carcinoma patients treated with sorafenib (V体育官网)
"VSports app下载" Abstract
Introduction: Sorafenib is an oral multikinase inhibitor that targets Raf kinase and receptor tyrosine kinases and has led to a longer median overall survival (OS) time and time to progression (TTP) in patients with advanced hepatocellular carcinoma (HCC). This study was conducted to assess the link between the antitumor efficacy of sorafenib and its early cutaneous side effects in advanced HCC patients. VSports手机版.
Materials and methods: All patients received 800 mg daily of sorafenib until progression or unacceptable toxicities. We retrospectively analyzed the incidence of rash and hand-foot skin reactions (HFSR) during the first month of treatment, comparing tumor control (partial response plus stable disease) and TTP V体育安卓版. .
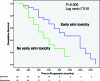
Results: Sixty-five HCC patients treated with sorafenib were included in this analysis: 47 (73. 3%) received sorafenib after failure of some local treatment, whereas 18 (27. 7%) received it as first-line treatment. Twenty-nine patients developed at least grade 1 skin toxicity (rash, 13; HFSR, 16). In patients who developed skin toxicity, the tumor control rate was 48. 3%, versus 19. 4% in patients without cutaneous side effects. The median TTP was 8. 1 months in the group of patients with skin toxicity versus 4. 0 months in those without skin toxicity. This difference was also statistically significant on multivariate analysis. A borderline statistically significant difference was also observed in terms of OS in patients with early skin toxicity. V体育ios版.
Conclusions: Skin toxicity should be closely monitored in HCC patients treated with sorafenib in relation to its potential role as a surrogate marker of efficacy VSports最新版本. .
Conflict of interest statement
The content of this article has been reviewed by independent peer reviewers to ensure that it is balanced, objective, and free from commercial bias. No financial relationships relevant to the content of this article have been disclosed by the authors or independent peer reviewers VSports注册入口.
Figures
References
-
- Wilhelm SM, Carter C, Tang L, et al. BAY 43–9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–7109. - PubMed
-
- Moreno-Aspitia A, Morton RF, Hillman DW, et al. Phase II trial of sorafenib in patients with metastatic breast cancer previously exposed to anthracyclines or taxanes: North Central Cancer Treatment Group and Mayo Clinic Trial N0336. J Clin Oncol. 2009;27:11–15. - "V体育官网入口" PMC - PubMed
-
- Abou-Alfa GK, Schwartz L, Ricci S, et al. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006;24:4293–4300. - PubMed
-
- Chu D, Lacouture ME, Fillos T, et al. Risk of hand-foot skin reaction with sorafenib: A systematic review and meta-analysis. Acta Oncol. 2008;47:176–186. - PubMed
-
- Beldner M, Jacobson M, Burges GE, et al. Localized palmar-plantar epidermal hyperplasia: A previously undefined dermatologic toxicity to sorafenib. The Oncologist. 2007;12:1178–1182. - V体育2025版 - PubMed
"VSports" MeSH terms
- V体育安卓版 - Actions
- "V体育官网" Actions
- "VSports在线直播" Actions
- VSports - Actions
- V体育安卓版 - Actions
- Actions (VSports手机版)
- "VSports" Actions
- V体育官网 - Actions
- "V体育ios版" Actions
- VSports app下载 - Actions
- VSports app下载 - Actions
Substances
- Actions (VSports在线直播)
- "VSports在线直播" Actions
- "VSports手机版" Actions
LinkOut - more resources
Full Text Sources
VSports注册入口 - Medical
Research Materials
Miscellaneous