Streptococcus pneumoniae evades human dendritic cell surveillance by pneumolysin expression
- PMID: 20049723
- PMCID: PMC3378134 (VSports最新版本)
- DOI: 10.1002/emmm.200900025
Streptococcus pneumoniae evades human dendritic cell surveillance by pneumolysin expression
Abstract (V体育平台登录)
Dendritic cells (DCs) protect the respiratory epithelium via induction of innate immune responses and priming of naïve T cells during the initiation of adaptive immunity. Streptococcus pneumoniae, a commonly carried asymptomatic member of the human nasopharyngeal microflora, can cause invasive and inflammatory diseases and the cholesterol-dependent cytotoxin pneumolysin is a major pneumococcal virulence factor implicated in compounding tissue damage and mediating inflammatory responses. While most studies examining the impact of pneumolysin have been based on murine models, we have focused this study on human DC responses. We show that expression of haemolytic pneumolysin inhibits human DC maturation, induction of proinflammatory cytokines and activation of the inflammasome. Furthermore, intracellular production of pneumolysin induces caspase-dependent apoptosis in infected DCs. Similarly, clinical isolates with non-haemolytic pneumolysin were more proinflammatory and caused less apoptosis compared to clonally related strains with active pneumolysin VSports手机版. This study describes a novel role of pneumolysin in the evasion of human DC surveillance that could have a profound clinical impact upon inflammatory disease progression and highlights the need to study human responses to human-specific pathogens. .
Figures

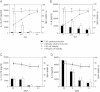
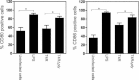
Statistical analysis of triplicate experiments revealed that T4R induced more apoptosis of human DCs than T4 as monitored by Annexin V-FITC positive cells (early apoptotic), Annexin V and PI positive cells (late-stage apoptotic) and PI positive cells (necrotic) V体育平台登录. Also, pneumolysin expression increased apoptosis irrespective of capsular expression. Mean (SE) is shown numerically and data is a compilation of three independent experiments. Significant changes compared to the parent strains (T4 and T4R) are indicated by p values. At least 5000 cells per treatment were counted by flow cytometry.
Inhibition of uptake of T4R with cytochalasin D and wortmannin (CW) decreased cell death VSports注册入口. Data represents mean + SE from three independent experiments.
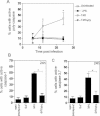
The effect of pneumolysin expression on the production of IL-12 p70 was examined following infection with live unencapsulated pneumococci at various MOIs. The production of IL-12 p70 was compared to cell viability by analysing the percentage of cells that stained negative for Annexin V and PI with flow cytometry. Data show mean ± SE (n = 3). Significant increases compared to T4R are indicated by an asterisk (*) for cytokines and a double asterisk (**) for cell viability.
IL-8 production and cell viability was also examined following infection with live unencapsulated pneumococci at various MOIs. Data show mean ± SE (n = 3). Significant increases compared to T4R are indicated by an asterisk (*) for cytokines and a double asterisk (**) for cell viability.
No effect of pneumolysin following infection with UV-killed pneumococci on the production of IL-12 p70 was observed. Data show mean ± SE (n = 3).
Pneumolysin did not affect IL-8 or cell viability following infection with UV-killed pneumococci. Data represent mean ± SE (n = 3).
 "VSports在线直播"
"VSports在线直播"
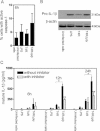
Pneumolysin-proficient pneumococci significantly activated multiple caspases at 12 and 24 h post-infection. Data represents mean ± SE (n = 3). Significant increases compared to uninfected cells are indicated by an asterisk (*).
Activation of caspase-1 at 24 h post-infection could also be attributed to pneumolysin expression. Data show mean + SE (n = 3) with significant differences indicated by an asterisk (*). 5000 events per treatment were counted by flow cytometry.
The activation of caspase 3,7 at 24 h post-infection could be attributed to pneumolysin expression. Data represent mean + SE (n = 3) and significant differences are indicated by an asterisk (*). 5000 events per treatment were counted by flow cytometry.
Pneumolysin-deficient pneumococci activated caspase-1 at 6 h post-infection, albeit at non-significant levels. Data represent the mean + SE (n = 3). At least 5000 events per treatment were counted.
In the absence of pneumolysin expression an increase in pro-IL-1β could be detected by Western blotting. Data is representative of three independent human DC preparations.
Pneumolysin-deficient pneumococci enhanced secreted levels of mature IL-1β as early as 6 h post-infection and addition of the caspase-1 inhibitor Z-YVAD-FMK inhibited this secretion. Data represent the mean + SE (n = 3). A single asterisk (*) shows significant increases compared to T4R and a double asterisk (**) indicates significant decreases compared to values obtained without the inhibitor.
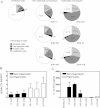
DCs were infected for 24 h with invasive serotype 1 pneumococcal clinical isolates and stained with Annexin V-FITC and PI. Flow cytometry revealed that the isolates of ST217 (BHN 166), ST228 (BHN 32) and ST227 (BHN 418) which express haemolytic pneumolysin induced apoptosis, whereas isolates of ST306 (BHN 31, BHN 339 and BHN 349) which express non-haemolytic pneumolysin did not induce apoptosis. At least 5000 cells per treatment were counted by flow cytometry and data shows mean (SE) numerically (n = 3).
Strains expressing non-haemolytic pneumolysin showed inhibited opsonised uptake. Data show mean + SE (n = 3).
Strains expressing non-haemolytic pneumolysin increased IL-12 p70 induction. Data represent mean + SE (n = 3) and significant induction of IL-12 p70 is indicated by an asterisk (*).
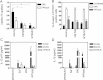
Human DCs showed enhanced uptake of pneumococci compared to murine BMDCs (BALB/c and C57BL/6). Data represent mean + SE. Significant decreases compared to human DCs are indicated by a single asterisk (*) and significant decreases compared to T4R are indicated by a double asterisk (**). Data comes from several independent experiments (human n = 10, BALB/c n = 4 and C57BL/6 n = 6).
Murine BMDCs underwent similar apoptosis to human DCs following exposure to pneumolysin-proficient pneumococci, as detected by Annexin V-FITC and PI staining. Data represent mean + SE (n = 3). Significant decreases compared to T4R infected cells are shown with a double asterisk (**). At least 5000 cells per treatment were counted by flow cytometry.
Human DCs showed increased production of the proinflammatory cytokine IL-12 p70 compared to BMDCs following pneumococcal infection. Data show mean + SE (n = 4). Significant increases compared to T4R infection are indicated by a single asterisk (*).
Human DCs showed altered production of the proinflammatory cytokine IL-1β compared to BMDCs following pneumococcal infection. Data represent mean + SE (n = 4). Significant increases compared to T4R infection are indicated by a single asterisk (*) and significant decreases (p < 0.05) are indicated by a double asterisk (**).
References
-
- Birmingham CL, Canadien V, Kaniuk NA, Steinberg BE, Higgins DE, Brumell JH. Listeriolysin O allows Listeria monocytogenes replication in macrophage vacuoles. Nature. 2008;451:350–354. - PubMed
-
- Brueggemann AB, Spratt BG. Geographic distribution and clonal diversity of Streptococcus pneumoniae serotype 1 isolates. J Clin Microbiol. 2003;41:4966–4970. - "V体育官网入口" PMC - PubMed
-
- Byington CL, Spencer LY, Johnson TA, Pavia AT, Allen D, Mason EO, Kaplan S, Carroll KC, Daly JA, Christenson JC, et al. An epidemiological investigation of a sustained high rate of pediatric parapneumonic empyema: risk factors and microbiological associations. Clin Infect Dis. 2002;34:434–440. - VSports最新版本 - PubMed
"V体育安卓版" Publication types
MeSH terms
- V体育平台登录 - Actions
- Actions (VSports在线直播)
- V体育安卓版 - Actions
- VSports app下载 - Actions
- V体育官网 - Actions
- Actions (VSports)
- Actions (V体育官网)
- VSports手机版 - Actions
- "VSports" Actions
- V体育官网入口 - Actions
- Actions (VSports app下载)
- "VSports注册入口" Actions
Substances (V体育官网入口)
- Actions (VSports在线直播)
- Actions (V体育2025版)
"VSports" LinkOut - more resources
Full Text Sources
Other Literature Sources (VSports最新版本)
Medical (VSports在线直播)

