"V体育2025版" Depletion of liver Kupffer cells prevents the development of diet-induced hepatic steatosis and insulin resistance
- PMID: 19934001
- PMCID: PMC2809951
- DOI: 10.2337/db09-0016
"VSports最新版本" Depletion of liver Kupffer cells prevents the development of diet-induced hepatic steatosis and insulin resistance
Abstract
Objective: Increased activity of the innate immune system has been implicated in the pathogenesis of the dyslipidemia and insulin resistance associated with obesity and type 2 diabetes. In this study, we addressed the potential role of Kupffer cells (liver-specific macrophages, KCs) in these metabolic abnormalities VSports手机版. .
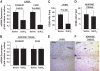
Research design and methods: Rats were depleted of KCs by administration of gadolinium chloride, after which all animals were exposed to a 2-week high-fat or high-sucrose diet. Subsequently, the effects of these interventions on the development of hepatic insulin resistance and steatosis were assessed V体育安卓版. In further studies, the effects of M1-polarized KCs on hepatocyte lipid metabolism and insulin sensitivity were addressed. .
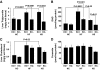
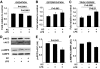
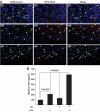
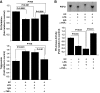
Results: As expected, a high-fat or high-sucrose diet induced steatosis and hepatic insulin resistance V体育ios版. However, these metabolic abnormalities were prevented when liver was depleted of KCs. In vitro, KCs recapitulated the in vivo effects of diet by increasing hepatocyte triglyceride accumulation and fatty acid esterification, and decreasing fatty acid oxidation and insulin responsiveness. To address the mechanisms(s) of KC action, we inhibited a panel of cytokines using neutralizing antibodies. Only neutralizing antibodies against tumor necrosis factor-alpha (TNFalpha) attenuated KC-induced alterations in hepatocyte fatty acid oxidation, triglyceride accumulation, and insulin responsiveness. Importantly, KC TNFalpha levels were increased by diet in vivo and in isolated M1-polarized KCs in vitro. .
Conclusions: These data demonstrate a role for liver macrophages in diet-induced alterations in hepatic lipid metabolism and insulin sensitivity, and suggest a role for these cells in the etiology of the metabolic abnormalities of obesity/type 2 diabetes VSports最新版本. .
Figures

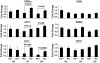

References
-
- Shoelson SE: Banking on ATM as a new target in metabolic syndrome. Cell Metab 2006;4:337–338 - PubMed
-
- Shoelson SE, Lee J, Goldfine AB: Inflammation and insulin resistance. J Clin Invest 2006;116:1793–1801 - V体育2025版 - PMC - PubMed
-
- Medzhitov R: Origin and physiological roles of inflammation. Nature 2008;454:428–435 - PubMed
VSports app下载 - Publication types
"V体育安卓版" MeSH terms
- Actions (V体育官网入口)
- V体育平台登录 - Actions
- Actions (V体育2025版)
- VSports在线直播 - Actions
- Actions (VSports app下载)
- "V体育安卓版" Actions
- Actions (V体育安卓版)
- Actions (V体育官网)
- "V体育平台登录" Actions
Substances
- "V体育平台登录" Actions
- V体育官网入口 - Actions
- "V体育官网" Actions
- Actions (VSports app下载)
- Actions (V体育2025版)
"V体育2025版" Grants and funding
LinkOut - more resources
"V体育ios版" Full Text Sources
"VSports" Other Literature Sources
Research Materials

