Characterisation of the proximal airway squamous metaplasia induced by chronic tobacco smoke exposure in spontaneously hypertensive rats
- PMID: 19930705
- PMCID: PMC2789729 (V体育2025版)
- DOI: 10.1186/1465-9921-10-118
"VSports在线直播" Characterisation of the proximal airway squamous metaplasia induced by chronic tobacco smoke exposure in spontaneously hypertensive rats
Abstract
Background: Continuous exposure to tobacco smoke (TS) is a key cause of chronic obstructive pulmonary disease (COPD), a complex multifactorial disease that is difficult to model in rodents. The spontaneously hypertensive (SH) rat exhibits several COPD-associated co-morbidities such as hypertension and increased coagulation. We have investigated whether SH rats are a more appropriate animal paradigm of COPD. VSports手机版.
Methods: SH rats were exposed to TS for 6 hours/day, 3 days/week for 14 weeks, and the lung tissues examined by immunohistochemistry V体育安卓版. .
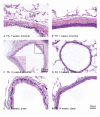
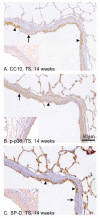
Results: TS induced a CK13-positive squamous metaplasia in proximal airways, which also stained for Ki67 and p63. We hypothesise that this lesion arises by basal cell proliferation, which differentiates to a squamous cell phenotype. Differences in staining profiles for the functional markers CC10 and surfactant D, but not phospho-p38, indicated loss of ability to function appropriately as secretory cells. Within the parenchyma, there were also differences in the staining profiles for CC10 and surfactant D, indicating a possible attempt to compensate for losses in proximal airways. In human COPD sections, areas of CK13-positive squamous metaplasia showed sporadic p63 staining, suggesting that unlike the rat, this is not a basal cell-driven lesion. V体育ios版.
Conclusion: This study demonstrates that although proximal airway metaplasia in rat and human are both CK13+ and therefore squamous, they potentially arise by different mechanisms VSports最新版本. .
Figures





References
-
- Kim V, Rogers TJ, Criner GJ. New Concepts in the Pathobiology of Chronic Obstructive Pulmonary Disease. Proc Am Thorac Soc. 2008;5:478–485. doi: 10.1513/pats.200802-014ET. - "VSports" DOI - PMC - PubMed
-
- Rennard SI. Inflammation and Repair Processes in Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 1999;160:12S–16. - "V体育安卓版" PubMed
Publication types
- Actions (V体育ios版)
- Actions (VSports注册入口)
"V体育官网入口" MeSH terms
- VSports最新版本 - Actions
- "V体育安卓版" Actions
- Actions (VSports手机版)
- "V体育ios版" Actions
- VSports最新版本 - Actions
- "V体育平台登录" Actions
- "VSports注册入口" Actions
- V体育官网入口 - Actions
- V体育安卓版 - Actions
- Actions (VSports在线直播)
- Actions (VSports)
- "VSports最新版本" Actions
- VSports - Actions
- "V体育官网入口" Actions
- Actions (V体育官网入口)
- "VSports在线直播" Actions
- Actions (VSports注册入口)
"VSports在线直播" Substances
- Actions (VSports手机版)
- Actions (VSports在线直播)
- "VSports在线直播" Actions
- "VSports在线直播" Actions
- VSports注册入口 - Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous (V体育安卓版)

