Chimeric NKG2D expressing T cells eliminate immunosuppression and activate immunity within the ovarian tumor microenvironment
- PMID: 19915047
- PMCID: PMC2825039
- DOI: 10.4049/jimmunol.0902000
Chimeric NKG2D expressing T cells eliminate immunosuppression and activate immunity within the ovarian tumor microenvironment
V体育平台登录 - Erratum in
- J Immunol. 2014 Aug 1;193(3):1513
Abstract
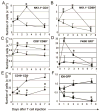
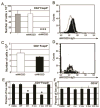
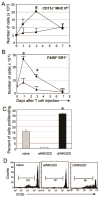
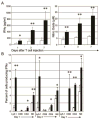
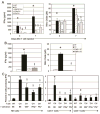
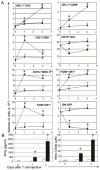
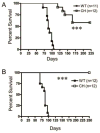
Adoptive transfer of T cells expressing chimeric NKG2D (chNKG2D) receptors, a fusion of NKG2D and CD3zeta, can lead to long-term, tumor-free survival in a murine model of ovarian cancer. To determine the mechanisms of chNKG2D T cell antitumor efficacy, we analyzed how chNKG2D T cells altered the tumor microenvironment, including the tumor-infiltrating leukocyte populations. chNKG2D T cell treatment of mice bearing ID8 tumor cells increased the number and activation of NK cells and increased the activation of host CD8+ T cells within the tumor. Foxp3+ regulatory T cells at the tumor site decreased more than 300-fold after chNKG2D T cell treatment. Tumor-associated regulatory T cells expressed cell surface NKG2D ligands and were killed by chNKG2D T cells in a perforin-dependent manner. chNKG2D T cells also altered the function of myeloid cells at the tumor site, changing these cells from being immunosuppressive to enhancing T cell responses. Cells isolated from the tumor produced elevated amounts of IFN-gamma, NO, and other proinflammatory cytokines after chNKG2D T cell treatment. ChNKG2D T cells required perforin, IFN-gamma, and GM-CSF to induce a full response at the tumor site. In addition, transfer of chNKG2D T cells into mice bearing tumors that were established for 5 weeks led to long-term survival of the mice. Thus, chNKG2D T cells altered the ovarian tumor microenvironment to eliminate immunosuppressive cells and induce infiltration and activation of antitumor immune cells and production of inflammatory cytokines VSports手机版. This induction of an immune response likely contributes to chNKG2D T cells' ability to eliminate established tumors. .
Figures
References
-
- Kershaw MH, Westwood JA, Parker LL, Wang G, Eshhar Z, Mavroukakis SA, White DE, Wunderlich JR, Canevari S, Rogers-Freezer L, Chen CC, Yang JC, Rosenberg SA, Hwu P. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res. 2006;12:6106–6115. - "VSports app下载" PMC - PubMed
-
- Nelson BH. The impact of T-cell immunity on ovarian cancer outcomes. Immunol Rev. 2008;222:101–116. - PubMed
Publication types
- "V体育安卓版" Actions
MeSH terms
- "V体育ios版" Actions
- Actions (VSports)
- V体育官网 - Actions
- "VSports注册入口" Actions
- Actions (V体育官网)
- Actions (VSports手机版)
- VSports注册入口 - Actions
- VSports app下载 - Actions
- Actions (V体育平台登录)
- Actions (V体育平台登录)
Substances
- "VSports注册入口" Actions
- V体育2025版 - Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

