"VSports手机版" Blocking LFA-1 activation with lovastatin prevents graft-versus-host disease in mouse bone marrow transplantation
- PMID: 19896074
- PMCID: PMC4809421
- DOI: 10.1016/j.bbmt.2009.08.013
"V体育2025版" Blocking LFA-1 activation with lovastatin prevents graft-versus-host disease in mouse bone marrow transplantation
Abstract
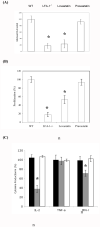
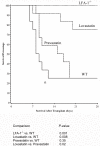
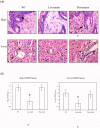
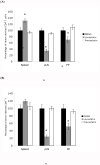
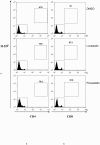
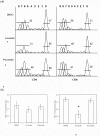
Graft-versus-host disease (GVHD) following bone marrow transplantation (BMT) is mediated by alloreactive donor T lymphocytes VSports手机版. Migration and activation of donor-derived T lymphocytes play critical roles in the development of GVHD. Leukocyte function-associated antigen-1 (LFA-1) regulates T cell adhesion and activation. We previously demonstrated that the I-domain, the ligand-binding site of LFA-1, changes from the low-affinity state to the high-affinity state on LFA-1 activation. Therapeutic antagonists, such as statins, inhibit LFA-1 activation and immune responses by modulating the affinity state of the LFA-1 I-domain. In the present study, we report that lovastatin blocked mouse T cell adhesion, proliferation, and cytokine production in vitro. Furthermore, blocking LFA-1 in the low-affinity state with lovastatin reduced the mortality and morbidity associated with GVHD in a murine BMT model. Specifically, lovastatin prevented T lymphocytes from homing to lymph nodes and Peyer's patches during the GVHD initiation phase and after donor lymphocyte infusion (DLI) after the establishment of GVHD. In addition, treatment with lovastatin impaired donor-derived T cell proliferation in vivo. Taken together, these results indicate the important role of lovastatin in the treatment of GVHD. .
Figures
"V体育安卓版" References
-
- Sale GE, Shulman HM, Hackman RC. Pathology of hematopoietic cell transplantation. In: Blume KG, Forman SJ, Appelbaum FR, editors. Thomas’s Hematopoietic Cell Transplantation. Blackwell Scientific Publications; Boston, MA: 2004. pp. 286–299.
-
- Antin JH, Deeg HJ. Clinical spectrum of acute graft-vs.-host disease. In: Ferrara JLM, Cooke KR, Deeg HJ, editors. Graft-vs.-Host-Disease. Marcel Dekker; New York, NY: 2005. pp. 369–382.
-
- Ferrara JLM, Cooke KR, Teshima T. The Pathophysiology of graft-vs.-host disease. In: Ferrara JLM, Cooke KR, Deeg HJ, editors. Graft-vs.-Host-Disease. Marcel Dekker; New York, NY: 2005. pp. 1–34.
-
- Korngold R, Friedman TM. Murine models for graft-versus-host disease. In: Blume KG, Forman SJ, Appelbaum FR, editors. Thomas’s Hematopoietic Cell Transplantation. Blackwell Scientific Publications; Boston, MA: 2004. pp. 353–368.
-
- Blazar BR, Korngold R, Vallera DA. Recent advances in graft-versus-host disease (GVHD) prevention. Immunol Rev. 1997;57:79–109. - "V体育官网" PubMed
MeSH terms (V体育2025版)
- Actions (VSports最新版本)
- Actions (VSports app下载)
- V体育平台登录 - Actions
- "V体育官网" Actions
- Actions (VSports最新版本)
- "V体育官网" Actions
- Actions (VSports app下载)
- "V体育2025版" Actions
- "V体育安卓版" Actions
- Actions (V体育安卓版)
- "V体育2025版" Actions
- V体育2025版 - Actions
Substances
- "VSports手机版" Actions
Grants and funding
LinkOut - more resources (VSports最新版本)
V体育官网 - Full Text Sources
Other Literature Sources
Medical

