"V体育官网入口" AAV recombineering with single strand oligonucleotides
- PMID: 19888330
- PMCID: PMC2765622
- DOI: V体育官网 - 10.1371/journal.pone.0007705
AAV recombineering with single strand oligonucleotides
"V体育安卓版" Abstract
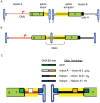
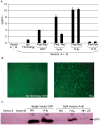
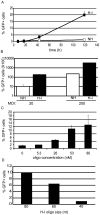
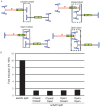
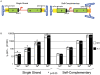
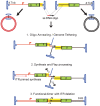
Adeno-associated virus (AAV) transduction initiates a signaling cascade that culminates in a transient DNA damage response. During this time, host DNA repair proteins convert the linear single-strand AAV genomes to double-strand circular monomers and concatemers in processes stimulated by the AAV inverted terminal repeats (ITRs). As the orientation of AAV genome concatemerization appears unbiased, the likelihood of concatemerization in a desired orientation is low (less than 1 in 6). Using a novel recombineering method, Oligo-Assisted AAV Genome Recombination (OAGR), this work demonstrates the ability to direct concatemerization specifically to a desired orientation in human cells. This was achieved by a single-strand DNA oligonucleotide (oligo) displaying homology to distinct AAV genomes capable of forming an intermolecular bridge for recombination. This DNA repair process results in concatemers with genomic junctions corresponding to the sequence of oligo homology. Furthermore, OAGR was restricted to single-strand, not duplexed, AAV genomes suggestive of replication-dependent recombination. Consistent with this process, OAGR demonstrated oligo polarity biases in all tested configurations except when a portion of the oligo targeted the ITR VSports手机版. This approach, in addition to being useful for the elucidation of intermolecular homologous recombination, may find eventual relevance for AAV mediated large gene therapy. .
V体育平台登录 - Conflict of interest statement
"VSports最新版本" Figures
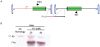
References
-
- Srivastava A, Lusby EW, Berns KI. Nucleotide sequence and organization of the adeno-associated virus 2 genome. J Virol. 1983;45:555–64. - "VSports注册入口" PMC - PubMed
-
- Ferrari FK, Samulski T, Shenk T, Samulski RJ. Second-strand synthesis is a rate-limiting step for efficient transduction by recombinant adeno-associated virus vectors. J Virol. 1996;70:3227–34. - "VSports手机版" PMC - PubMed
-
- Duan D, Yue Y, Engelhardt JF. Expanding AAV packaging capacity with trans-splicing or overlapping vectors: a quantitative comparison. Mol Ther. 2001;4:383–91. - VSports注册入口 - PubMed
"VSports app下载" Publication types
- VSports app下载 - Actions
"V体育安卓版" MeSH terms
- VSports app下载 - Actions
- Actions (VSports app下载)
- V体育平台登录 - Actions
- VSports在线直播 - Actions
Substances
Grants and funding
LinkOut - more resources (VSports手机版)
Full Text Sources
Research Materials

