Dendritic cells for active anti-cancer immunotherapy: targeting activation pathways through genetic modification (V体育官网)
- PMID: 19857199
- PMCID: "V体育2025版" PMC2797000
- DOI: 10.2174/187153009789839156
V体育2025版 - Dendritic cells for active anti-cancer immunotherapy: targeting activation pathways through genetic modification
Abstract
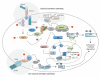
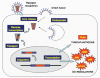
Tumour immunotherapy has become a treatment modality for cancer, harnessing the immune system to recognize and eradicate tumour cells specifically. It is based on the expression of tumour associated antigens (TAA) by the tumour cells and aims at the induction of TAA-specific effector T cell responses, whilst overruling various mechanisms that can hamper the anti-tumour immune response, e. g. regulatory T cells (Treg). (Re-) activation of effector T cells requires the completion of a carefully orchestrated series of specific steps VSports手机版. Particularly important is the provision of TAA presentation and strong stimulatory signals, delivered by co-stimulatory surface molecules and cytokines. These can only be delivered by professional antigen-presenting cells, in particular dendritic cells (DC). Therefore, DC need to be loaded with TAA and appropriately activated. It is not surprising that an extensive part of DC research has focused on the delivery of both TAA and activation signals to DC, developing a one step approach to obtain potent stimulatory DC. The simultaneous delivery of TAA and activation signals is therefore the topic of this review, emphasizing the role of DC in mediating T cell activation and how we can manipulate DC for the pill-pose of enhancing tumour immunotherapy. As we gain a better understanding of the molecular and cellular mechanisms that mediate induction of TAA-specific T cells, rational approaches for the activation of T cell responses can be developed for the treatment of cancer. .
Figures

"V体育2025版" References
-
- Shaw GB. The Doctor’s Dilemma. Int. J. Epidemiol. 2003;32:910–915. - PubMed (V体育平台登录)
-
- Ehrlich P. Ueber den jetzigen Stand der Karzinomforschung. Ned. Tijdschr. Geneesk. 1909;5:273–290.
-
- Mak TW. The T cell antigen receptor: “The Hunting of the Snark”. Eur. J. Immunol. 2007;37:S83–S93. - PubMed
-
- Burnet FM. The concept of immunological surveillance. Prog. Exp. Tumor Res. 1970;13:1–27. - "VSports app下载" PubMed
Publication types
MeSH terms
- Actions (VSports app下载)
- Actions (VSports注册入口)
- Actions (VSports注册入口)
- VSports在线直播 - Actions
- "V体育官网入口" Actions
- Actions (V体育2025版)
- "VSports" Actions
- Actions (V体育官网入口)
- Actions (V体育2025版)
- V体育ios版 - Actions
Substances
- "V体育2025版" Actions
Grants and funding
LinkOut - more resources
"VSports最新版本" Full Text Sources
Other Literature Sources
Medical
Research Materials

