Early termination of ISRCTN45828668, a phase 1/2 prospective, randomized study of sulfasalazine for the treatment of progressing malignant gliomas in adults
- PMID: 19840379
- PMCID: PMC2771045
- DOI: 10.1186/1471-2407-9-372
Early termination of ISRCTN45828668, a phase 1/2 prospective, randomized study of sulfasalazine for the treatment of progressing malignant gliomas in adults
Abstract
Background: Sulfasalazine, a NF-kappaB and x(c)-cystine/glutamate antiport inhibitor, has demonstrated a strong antitumoral potential in preclinical models of malignant gliomas. As it presents an excellent safety profile, we initiated a phase 1/2 clinical study of this anti-inflammatory drug for the treatment of recurrent WHO grade 3 and 4 astrocytic gliomas in adults VSports手机版. .
Methods: 10 patients with advanced recurrent anaplastic astrocytoma (n = 2) or glioblastoma (n = 8) aged 32-62 years were recruited prior to the planned interim analysis of the study. Subjects were randomly assigned to daily doses of 1. 5, 3, 4 V体育安卓版. 5, or 6 grams of oral sulfasalazine, and treated until clinical or radiological evidence of disease progression or the development of serious or unbearable side effects. Primary endpoints were the evaluation of toxicities according to the CTCAE v. 3. 0, and the observation of radiological tumor responses based on MacDonald criteria. .
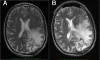
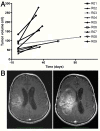
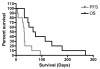
Results: No clinical response was observed V体育ios版. One tumor remained stable for 2 months with sulfasalazine treatment, at the lowest daily dose of the drug. The median progression-free survival was 32 days. Side effects were common, as all patients developed grade 1-3 adverse events (mean: 7. 2/patient), four patients developed grade 4 toxicity. Two patients died while on treatment or shortly after its discontinuation. .
Conclusion: Although the proper influence of sulfasalazine treatment on patient outcome was difficult to ascertain in these debilitated patients with a large tumor burden (median KPS = 50), ISRCTN45828668 was terminated after its interim analysis. This study urges to exert cautiousness in future trials of Sulfasalazine for the treatment of malignant gliomas. VSports最新版本.
Trial registration: Current Controlled Trials ISRCTN45828668. V体育平台登录.
Figures
References
-
- Robe PA, Bentires-Alj M, Bonif M, Rogister B, Deprez M, Haddada H, Khac MT, Jolois O, Erkmen K, Merville MP. In vitro and in vivo activity of the nuclear factor-kappaB inhibitor sulfasalazine in human glioblastomas. Clin Cancer Res. 2004;10(16):5595–5603. doi: 10.1158/1078-0432.CCR-03-0392. - DOI - PubMed
-
- Manna SK, Aggarwal BB. Alpha-melanocyte-stimulating hormone inhibits the nuclear transcription factor NF-kappa B activation induced by various inflammatory agents. J Immunol. 1998;161(6):2873–2880. - PubMed
Publication types
- VSports手机版 - Actions
- Actions (VSports在线直播)
MeSH terms
- "VSports最新版本" Actions
- Actions (VSports在线直播)
- "V体育平台登录" Actions
- Actions (VSports注册入口)
- "V体育ios版" Actions
- Actions (VSports在线直播)
- "VSports" Actions
- "VSports app下载" Actions
- V体育ios版 - Actions
- "VSports app下载" Actions
- "V体育ios版" Actions
Substances
VSports注册入口 - Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials (V体育ios版)

