VSports手机版 - Protein phosphatase 1 regulates the histone code for long-term memory
- PMID: 19828821
- PMCID: VSports app下载 - PMC6665317
- DOI: 10.1523/JNEUROSCI.3610-09.2009
Protein phosphatase 1 regulates the histone code for long-term memory
Abstract (V体育2025版)
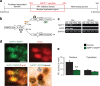
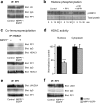
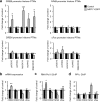
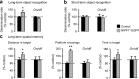
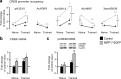
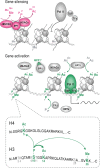
Chromatin remodeling through histone posttranslational modifications (PTMs) and DNA methylation has recently been implicated in cognitive functions, but the mechanisms involved in such epigenetic regulation remain poorly understood. Here, we show that protein phosphatase 1 (PP1) is a critical regulator of chromatin remodeling in the mammalian brain that controls histone PTMs and gene transcription associated with long-term memory. Our data show that PP1 is present at the chromatin in brain cells and interacts with enzymes of the epigenetic machinery including HDAC1 (histone deacetylase 1) and histone demethylase JMJD2A (jumonji domain-containing protein 2A). The selective inhibition of the nuclear pool of PP1 in forebrain neurons in transgenic mice is shown to induce several histone PTMs that include not only phosphorylation but also acetylation and methylation. These PTMs are residue-specific and occur at the promoter of genes important for memory formation like CREB (cAMP response element-binding protein) and NF-kappaB (nuclear factor-kappaB). These histone PTMs further co-occur with selective binding of RNA polymerase II and altered gene transcription, and are associated with improved long-term memory for objects and space. Together, these findings reveal a novel mechanism for the epigenetic control of gene transcription and long-term memory in the adult brain that depends on PP1. VSports手机版.
Figures

VSports注册入口 - References
-
- Alarcón JM, Malleret G, Touzani K, Vronskaya S, Ishii S, Kandel ER, Barco A. Chromatin acetylation, memory, and LTP are impaired in CBP+/− mice: a model for the cognitive deficit in Rubinstein-Taybi syndrome and its amelioration. Neuron. 2004;42:947–959. - PubMed
-
- Arthur JS. MSK activation and physiological roles. Front Biosci. 2008;13:5866–5879. - PubMed
-
- Bennett D. Transcriptional control by chromosome-associated protein phosphatase-1. Biochem Soc Trans. 2005;33:1444–1446. - PubMed
-
- Beullens M, Van Eynde A, Stalmans W, Bollen M. The isolation of novel inhibitory polypeptides of protein phosphatase 1 from bovine thymus nuclei. J Biol Chem. 1992;267:16538–16544. - PubMed
VSports最新版本 - Publication types
- Actions (V体育官网入口)
MeSH terms
- V体育官网入口 - Actions
- Actions (VSports在线直播)
- Actions (V体育ios版)
- Actions (VSports)
- Actions (VSports最新版本)
- "VSports手机版" Actions
- Actions (VSports)
- "VSports在线直播" Actions
- Actions (VSports最新版本)
- Actions (V体育ios版)
- VSports手机版 - Actions
- VSports app下载 - Actions
- "V体育官网" Actions
- "VSports" Actions
- "VSports手机版" Actions
Substances
- Actions (VSports手机版)
- Actions (V体育2025版)
- "VSports" Actions
- VSports最新版本 - Actions
- "VSports注册入口" Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical (VSports app下载)
Molecular Biology Databases
Miscellaneous
