Limited terminal transferase in human DNA polymerase mu defines the required balance between accuracy and efficiency in NHEJ
- PMID: 19805281
- PMCID: PMC2738622
- DOI: 10.1073/pnas.0908492106
Limited terminal transferase in human DNA polymerase mu defines the required balance between accuracy and efficiency in NHEJ
V体育安卓版 - Abstract
DNA polymerase mu (Polmu) is a family X member implicated in DNA repair, with template-directed and terminal transferase (template-independent) activities. It has been proposed that the terminal transferase activity of Polmu can be specifically required during non-homologous end joining (NHEJ) to create or increase complementarity of DNA ends. By site-directed mutagenesis in human Polmu, we have identified a specific DNA ligand residue (Arg(387)) that is responsible for its limited terminal transferase activity compared to that of human TdT, its closest homologue (42% amino acid identity). Polmu mutant R387K (mimicking TdT) displayed a large increase in terminal transferase activity, but a weakened interaction with ssDNA. That paradox can be explained by the regulatory role of Arg(387) in the translocation of the primer from a non-productive E:DNA complex to a productive E:DNA:dNTP complex in the absence of a templating base, which is probably the rate limiting step during template-independent synthesis VSports手机版. Further, we show that the Polmu switch from terminal transferase to templated insertions in NHEJ reactions is triggered by recognition of a 5'-P at a second DNA end, whose 3'-protrusion could provide a templating base to facilitate such a special "pre-catalytic translocation step. " These studies shed light on the mechanism by which a rate-limited terminal transferase activity in Polmu could regulate the balance between accuracy and necessary efficiency, providing some variability during NHEJ. .
Conflict of interest statement
The authors declare no conflict of interest.
Figures


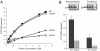


References
-
- Friedberg EC, et al. DNA Repair and Mutagenesis. 2nd Ed. Washington D.C.: ASM Press; 2006.
-
- Bebenek K, Kunkel TA. Functions of DNA polymerases. Adv Protein Chem. 2004;69:137–165. - PubMed
-
- Bork P, et al. A superfamily of conserved domains in DNA damage-responsive cell cycle checkpoint proteins. FASEB J. 1997;11:68–76. - PubMed
-
- Callebaut I, Mornon JP. From BRCA1 to RAP1: A widespread BRCT module closely associated with DNA repair. FEBS Lett. 1997;400:25–30. - PubMed
Publication types
MeSH terms (V体育官网入口)
- Actions (VSports在线直播)
- "VSports最新版本" Actions
- Actions (VSports手机版)
- Actions (VSports app下载)
- Actions (VSports手机版)
- Actions (VSports手机版)
- Actions (V体育官网入口)
- VSports在线直播 - Actions
- Actions (VSports app下载)
- Actions (VSports手机版)
- Actions (V体育官网)
- Actions (VSports注册入口)
- V体育官网入口 - Actions
- "VSports" Actions
"V体育2025版" Substances
- "V体育2025版" Actions
- VSports最新版本 - Actions
"V体育官网" LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

