Profiling protein thiol oxidation in tumor cells using sulfenic acid-specific antibodies
- PMID: 19805274
- PMCID: VSports在线直播 - PMC2741475
- DOI: 10.1073/pnas.0903015106
Profiling protein thiol oxidation in tumor cells using sulfenic acid-specific antibodies
Abstract
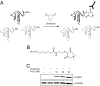
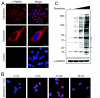
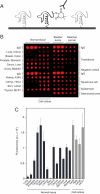
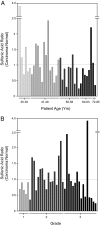
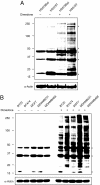
Hydrogen peroxide (H2O2) functions as a second messenger that can activate cell proliferation through chemoselective oxidation of cysteine residues in signaling proteins. The connection between H2O2 signaling, thiol oxidation, and activation of growth pathways has emerged as fertile ground for the development of strategies for cancer treatment. Central to achieving this goal is the development of tools and assays that facilitate characterization of the molecular events associated with tumorigenesis and evaluation of patient response to therapy. Here we report on the development of an immunochemical method for detecting sulfenic acid, the initial oxidation product that results when a thiolate reacts with H2O2. For this approach, the sulfenic acid is derivatized with a chemical tag to generate a unique epitope for recognition. The elicited antibody is exquisitely specific, context-independent, and capable of visualizing sulfenic acid formation in cells. Applying this approach to several systems, including cancer cell lines, shows it can be used to monitor differences in thiol redox status and reveals a diverse pattern of sulfenic acid modifications across different subtypes of breast tumors VSports手机版. These studies demonstrate a general strategy for producing antibodies against a specific oxidation state of cysteine and show the utility of these reagents for profiling thiol oxidation associated with pathological conditions such as breast cancer. .
Conflict of interest statement (V体育官网)
Conflict of interest statement: The University of Michigan is presently negotiating with Assay Designs to commercialize this antibody along with the necessary reagents for labeling protein sulfenic acids.
Figures
References
-
- Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. 4th Ed. New York: Oxford University Press; 2007. p. 851.
-
- Halliwell B. Oxidative stress and cancer: Have we moved forward? Biochem J. 2007;401:1–11. - PubMed
-
- Klaunig JE, Kamendulis LM. The role of oxidative stress in carcinogenesis. Annu Rev Pharmacol Toxicol. 2004;44:239–267. - PubMed
-
- Finkel T. Oxidant signals and oxidative stress. Curr Opin Cell Biol. 2003;15:247–254. - PubMed
Publication types
"VSports手机版" MeSH terms
- "VSports app下载" Actions
- "V体育ios版" Actions
- Actions (V体育平台登录)
- "V体育安卓版" Actions
- "VSports在线直播" Actions
- "VSports app下载" Actions
- V体育安卓版 - Actions
- "VSports app下载" Actions
- "V体育官网入口" Actions
- Actions (V体育平台登录)
- "V体育2025版" Actions
- V体育安卓版 - Actions
- V体育官网入口 - Actions
- Actions (VSports app下载)
- "VSports app下载" Actions
Substances
- "V体育平台登录" Actions
- "VSports手机版" Actions
- V体育官网 - Actions
- "VSports手机版" Actions
LinkOut - more resources (V体育官网)
Full Text Sources
Other Literature Sources (VSports最新版本)

