V体育平台登录 - The mercaptopyruvate sulfurtransferase of Trichomonas vaginalis links cysteine catabolism to the production of thioredoxin persulfide
- PMID: 19762467
- PMCID: PMC2785193
- DOI: 10.1074/jbc.M109.054320
The mercaptopyruvate sulfurtransferase of Trichomonas vaginalis links cysteine catabolism to the production of thioredoxin persulfide
Abstract
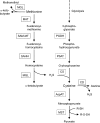
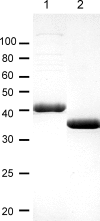
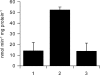
Trichomonas vaginalis is a protozoan parasite of humans that is able to synthesize cysteine de novo using cysteine synthase but does not produce glutathione. In this study, high pressure liquid chromatography analysis confirmed that cysteine is the major intracellular redox buffer by showing that T. vaginalis contains high levels of cysteine ( approximately 600 mum) comprising more than 70% of the total thiols detected. To investigate possible mechanisms for the regulation of cysteine levels in T. vaginalis, we have characterized enzymes of the mercaptopyruvate pathway. This consists of an aspartate aminotransferase (TvAspAT1), which transaminates cysteine to form 3-mercaptopyruvate (3-MP), and mercaptopyruvate sulfurtransferase (TvMST), which transfers the sulfur of 3-MP to a nucleophilic acceptor, generating pyruvate. TvMST has high activity with 3-MP as a sulfur donor and can use several thiol compounds as sulfur acceptor substrates. Our analysis indicated that TvMST has a k(cat)/K(m) for reduced thioredoxin of 6. 2 x 10(7) m(-1) s(-1), more than 100-fold higher than that observed for beta-mercaptoethanol and cysteine, suggesting that thioredoxin is a preferred substrate for TvMST. Thiol trapping and mass spectrometry provided direct evidence for the formation of thioredoxin persulfide as a product of this reaction. The thioredoxin persulfide could serve a biological function such as the transfer of the persulfide to a target protein or the sequestered release of sulfide for biosynthesis. Changes in MST activity of T VSports手机版. vaginalis in response to variation in the supply of exogenous cysteine are suggestive of a role for the mercaptopyruvate pathway in the removal of excess intracellular cysteine, redox homeostasis, and antioxidant defense. .
Figures

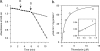
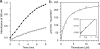
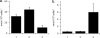
"V体育官网入口" References
-
- Cotch M. F., Pastorek J. G., 2nd, Nugent R. P., Hillier S. L., Gibbs R. S., Martin D. H., Eschenbach D. A., Edelman R., Carey J. C., Regan J. A., Krohn M. A., Klebanoff M. A., Rao A. V., Rhoads G. G. (1997) Sex. Transm. Dis. 24, 353–360 - "V体育安卓版" PubMed
-
- Schwebke J. R., Burgess D. (2004) Clin. Microbiol. Rev. 17, 794–803 - "VSports最新版本" PMC - PubMed
-
- McClelland R. S., Sangare L., Hassan W. M., Lavreys L., Mandaliya K., Kiarie J., Ndinya-Achola J., Jaoko W., Baeten J. M. (2007) J. Infect. Dis. 195, 698–702 - PubMed
-
- Van der Pol B., Kwok C., Pierre-Louis B., Rinaldi A., Salata R. A., Chen P. L., van de Wijgert J., Mmiro F., Mugerwa R., Chipato T., Morrison C. S. (2008) J. Infect. Dis. 197, 548–554 - PubMed
MeSH terms
- "V体育2025版" Actions
- Actions (V体育官网)
- "VSports手机版" Actions
- V体育安卓版 - Actions
- "VSports app下载" Actions
- "VSports最新版本" Actions
- "V体育ios版" Actions
- "VSports注册入口" Actions
- "V体育官网入口" Actions
- VSports app下载 - Actions
- Actions (V体育安卓版)
- "VSports app下载" Actions
V体育官网 - Substances
- "VSports最新版本" Actions
- V体育2025版 - Actions
- "V体育平台登录" Actions
- "VSports注册入口" Actions
"V体育官网入口" Grants and funding
LinkOut - more resources
V体育官网 - Full Text Sources
Other Literature Sources
Miscellaneous

