MKP-1 is necessary for T cell activation and function (V体育安卓版)
- PMID: 19748894
- PMCID: PMC2781480
- DOI: 10.1074/jbc.M109.052472
MKP-1 is necessary for T cell activation and function
Abstract
MAPKs are evolutionarily conserved immune regulators VSports手机版. MAPK phosphatases (MKPs) that negatively regulate MAPK activities have recently emerged as critical players in both innate and adaptive immune responses. MKP-1, also known as DUSP1, was previously shown to negatively regulate innate immunity by inhibiting pro-inflammatory cytokine production. Here, we found that MKP-1 is necessary in T cell activation and function. MKP-1 deficiency in T cells impaired the activation, proliferation, and function of T cells in vitro, associated with enhanced activation of JNK and reduced NFATc1 translocation into the nucleus. Consistently, MKP-1(-/-) mice were defective in anti-influenza immunity in vivo and resistant to experimental autoimmune encephalomyelitis. Our results thus demonstrate that MKP-1 is a critical positive regulator of T cell activation and function and may be targeted in treatment of autoimmune diseases. .
"V体育安卓版" Figures



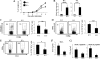
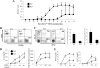
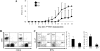
References
-
- Dong C., Davis R. J., Flavell R. A. (2002) Annu. Rev. Immunol. 20, 55–72 - PubMed
-
- Zhang Y., Blattman J. N., Kennedy N. J., Duong J., Nguyen T., Wang Y., Davis R. J., Greenberg P. D., Flavell R. A., Dong C. (2004) Nature 430, 793–797 - PubMed
-
- Jeffrey K. L., Brummer T., Rolph M. S., Liu S. M., Callejas N. A., Grumont R. J., Gillieron C., Mackay F., Grey S., Camps M., Rommel C., Gerondakis S. D., Mackay C. R. (2006) Nat. Immunol. 7, 274–283 - "VSports在线直播" PubMed
-
- Charles C. H., Abler A. S., Lau L. F. (1992) Oncogene 7, 187–190 - PubMed
-
- Keyse S. M., Emslie E. A. (1992) Nature 359, 644–647 - "V体育官网入口" PubMed
"V体育安卓版" Publication types
MeSH terms
- Actions (VSports最新版本)
- "VSports手机版" Actions
- "VSports最新版本" Actions
- "VSports注册入口" Actions
- "VSports app下载" Actions
- Actions (V体育官网入口)
- Actions (V体育平台登录)
Substances
- Actions (VSports手机版)
Grants and funding
LinkOut - more resources
Full Text Sources
"VSports" Other Literature Sources
Molecular Biology Databases
Research Materials
V体育安卓版 - Miscellaneous

