"VSports" Specific requirements for Vgamma9Vdelta2 T cell stimulation by a natural adenylated phosphoantigen
- PMID: 19710470
- PMCID: PMC2809082
- DOI: VSports注册入口 - 10.4049/jimmunol.0901085
Specific requirements for Vgamma9Vdelta2 T cell stimulation by a natural adenylated phosphoantigen
Abstract
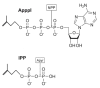
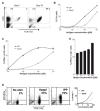
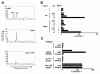
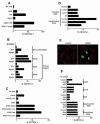
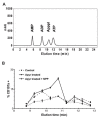
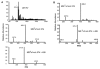
Human Vgamma9Vdelta2 T lymphocytes recognize phosphorylated alkyl Ags. Isopentenyl pyrophosphate (IPP) was previously proposed as the main Ag responsible for Vgamma9Vdelta2 T cell activation by cancer cells. However, triphosphoric acid 1-adenosin-5'-yl ester 3-(3-methylbut-3-enyl) ester (ApppI), a metabolite in which the isopentenyl moiety is linked to ATP, was reported in cells activated with aminobisphosphonates. The contribution of this compound to tumor-stimulatory activity was thus examined. ApppI induces selective expansion of Vgamma9Vdelta2 T cells from PBMCs. In the absence of APCs, however, ApppI has little stimulatory activity on Vgamma9Vdelta2 T cells, and optimal activation with ApppI requires addition of a nucleotide pyrophosphatase releasing IPP plus AMP. Thus, ApppI has no intrinsic stimulatory activity VSports手机版. Nevertheless, stimulation by ApppI is strengthened by the presence of APCs. Moreover, in contrast to IPP, ApppI can be efficiently pulsed on dendritic cells as well as on nonprofessional APCs. Pulsed APCs display stable and phosphatase-resistant stimulatory activity, indicative of Ag modification. HPLC analysis of tumor cell extracts indicates that latent phosphoantigenic activity is stored intracellularly in the Vgamma9Vdelta2 cell-sensitive tumor Daudi and can be activated by a nucleotide pyrophosphatase activity. The presence of ApppI in Daudi cell extracts was demonstrated by mass spectrometry. Nucleotidic Ags such as ApppI are thus a storage form of phosphoantigen which may represent a major source of phosphoantigenic activity in tumor cells. The unique properties of ApppI may be important for the design of Ags used in anticancer immunotherapeutic protocols using Vgamma9Vdelta2 cells. .
Figures

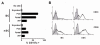
References
-
- Hayday AC. [gamma][delta] cells: a right time and a right place for a conserved third way of protection. Annu Rev Immunol. 2000;18:975–1026. - PubMed
-
- Kabelitz D, Wesch D, Pitters E, Zoller M. Potential of human gammadelta T lymphocytes for immunotherapy of cancer. Int J Cancer. 2004;112:727–732. - VSports - PubMed
-
- Dieli F, Vermijlen D, Fulfaro F, Caccamo N, Meraviglia S, Cicero G, Roberts A, Buccheri S, D’Asaro M, Gebbia N, Salerno A, Eberl M, Hayday AC. Targeting human {gamma}delta} T cells with zoledronate and interleukin-2 for immunotherapy of hormone-refractory prostate cancer. Cancer Res. 2007;67:7450–7457. - PMC - PubMed
-
- Constant P, Davodeau F, Peyrat MA, Poquet Y, Puzo G, Bonneville M, Fournie JJ. Stimulation of human gamma delta T cells by nonpeptidic mycobacterial ligands. Science. 1994;264:267–270. - VSports app下载 - PubMed
"VSports最新版本" Publication types
"VSports app下载" MeSH terms
- "VSports" Actions
- "VSports app下载" Actions
- V体育官网 - Actions
- "VSports注册入口" Actions
- VSports在线直播 - Actions
- "V体育安卓版" Actions
- Actions (V体育官网入口)
- VSports最新版本 - Actions
Substances
- V体育官网 - Actions
- Actions (V体育官网)
- "VSports" Actions
- "VSports手机版" Actions
- Actions (VSports注册入口)
- "VSports app下载" Actions
LinkOut - more resources
Full Text Sources
VSports手机版 - Miscellaneous

