Novel human interleukin-15 agonists
- PMID: 19710453
- PMCID: PMC2814526
- DOI: 10.4049/jimmunol.0901244
VSports在线直播 - Novel human interleukin-15 agonists
Abstract
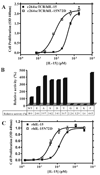
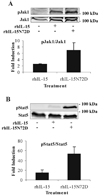
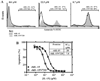
IL-15 is an immunostimulatory cytokine trans-presented with the IL-15 receptor alpha-chain to the shared IL-2/IL-15Rbeta and common gamma-chains displayed on the surface of T cells and NK cells. To further define the functionally important regions of this cytokine, activity and binding studies were conducted on human IL-15 muteins generated by site-directed mutagenesis. Amino acid substitutions of the asparagine residue at position 72, which is located at the end of helix C, were found to provide both partial agonist and superagonist activity, with various nonconservative substitutions providing enhanced activity. Particularly, the N72D substitution provided a 4-5-fold increase in biological activity of the IL-15 mutein compared with the native molecule based on proliferation assays with cells bearing human IL-15Rbeta and common gamma-chains. The IL-15N72D mutein exhibited superagonist activity through improved binding ability to the human IL-15Rbeta-chain. However, the enhanced potency of IL-15N72D was not observed with cells expressing the mouse IL-15Ralpha-IL-15Rbeta-gamma(c) complex, suggesting that this effect is specific to the human IL-15 receptor. The enhanced biological activity of IL-15N72D was associated with more intense phosphorylation of Jak1 and Stat5 and better anti-apoptotic activity compared with the wild-type IL-15 VSports手机版. IL-15N72D superagonist activity was also preserved when linked to a single-chain TCR domain to generate a tumor-specific fusion protein. Thus, the human IL-15 superagonist muteins and fusions may create opportunities to construct more efficacious immunotherapeutic agents with clinical utility. .
Figures (V体育2025版)




References
-
- Belmont HJ, Price-Schiavi S, Liu B, Card KF, Lee HI, Han KP, Wen J, Tang S, Zhu X, Merrill J, Chavillaz PA, Wong JL, Rhode PR, Wong HC. Potent antitumor activity of a tumor-specific soluble TCR/IL-2 fusion protein. Clin Immunol. 2006;121:29–39. - PubMed
-
- Wen J, Zhu X, Liu B, You L, Kong L, Lee HI, Han KP, Wong JL, Rhode PR, Wong HC. Targeting activity of a TCR/IL-2 fusion protein against established tumors. Cancer Immunol Immunother. 2008;57:1781–1794. - "VSports注册入口" PMC - PubMed
-
- Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6:595–601. - "VSports在线直播" PubMed
-
- Ma A, Koka R, Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu Rev Immunol. 2006;24:657–679. - PubMed
-
- Stoklasek TA, Schluns KS, Lefrancois L. Combined IL-15/IL-15Ralpha immunotherapy maximizes IL-15 activity in vivo. J Immunol. 2006;177:6072–6080. - "VSports手机版" PMC - PubMed
MeSH terms
- Actions (VSports)
- "V体育平台登录" Actions
- "V体育2025版" Actions
- "VSports" Actions
- Actions (VSports在线直播)
- Actions (V体育平台登录)
- Actions (V体育官网入口)
- Actions (VSports在线直播)
- Actions (VSports在线直播)
VSports手机版 - Substances
- VSports在线直播 - Actions
- Actions (VSports app下载)
- V体育安卓版 - Actions
- "V体育平台登录" Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources (V体育安卓版)
Molecular Biology Databases
V体育安卓版 - Research Materials
Miscellaneous

