Cortactin regulates cofilin and N-WASp activities to control the stages of invadopodium assembly and maturation
- PMID: 19704022
- PMCID: PMC2733743
- DOI: 10.1083/jcb.200812176
Cortactin regulates cofilin and N-WASp activities to control the stages of invadopodium assembly and maturation
Abstract
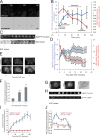
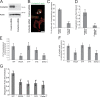
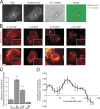
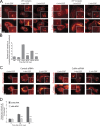
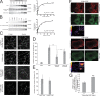
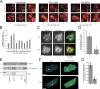
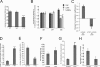
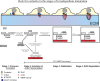
Invadopodia are matrix-degrading membrane protrusions in invasive carcinoma cells. The mechanisms regulating invadopodium assembly and maturation are not understood. We have dissected the stages of invadopodium assembly and maturation and show that invadopodia use cortactin phosphorylation as a master switch during these processes. In particular, cortactin phosphorylation was found to regulate cofilin and Arp2/3 complex-dependent actin polymerization. Cortactin directly binds cofilin and inhibits its severing activity. Cortactin phosphorylation is required to release this inhibition so cofilin can sever actin filaments to create barbed ends at invadopodia to support Arp2/3-dependent actin polymerization. After barbed end formation, cortactin is dephosphorylated, which blocks cofilin severing activity thereby stabilizing invadopodia VSports手机版. These findings identify novel mechanisms for actin polymerization in the invadopodia of metastatic carcinoma cells and define four distinct stages of invadopodium assembly and maturation consisting of invadopodium precursor formation, actin polymerization, stabilization, and matrix degradation. .
Figures (V体育2025版)

References
-
- Artym V.V., Zhang Y., Seillier-Moiseiwitsch F., Yamada K.M., Mueller S.C. 2006. Dynamic interactions of cortactin and membrane type 1 matrix metalloproteinase at invadopodia: defining the stages of invadopodia formation and function.Cancer Res. 66:3034–3043 - PubMed
-
- Ayala I., Baldassarre M., Giacchetti G., Caldieri G., Tetè S., Luini A., Buccione R. 2008. Multiple regulatory inputs converge on cortactin to control invadopodia biogenesis and extracellular matrix degradation.J. Cell Sci. 121:369–378 - PubMed (VSports最新版本)
-
- Boyle S.N., Michaud G.A., Schweitzer B., Predki P.F., Koleske A.J. 2007. A critical role for cortactin phosphorylation by Abl-family kinases in PDGF-induced dorsal-wave formation.Curr. Biol. 17:445–451 - PubMed (V体育安卓版)
-
- Bryce N.S., Clark E.S., Leysath J.L., Currie J.D., Webb D.J., Weaver A.M. 2005. Cortactin promotes cell motility by enhancing lamellipodial persistence.Curr. Biol. 15:1276–1285 - PubMed
Publication types
- Actions (VSports手机版)
MeSH terms (VSports最新版本)
- "V体育安卓版" Actions
- "VSports注册入口" Actions
- "V体育2025版" Actions
- Actions (V体育平台登录)
- V体育ios版 - Actions
- "V体育平台登录" Actions
- V体育平台登录 - Actions
- Actions (V体育平台登录)
- "VSports最新版本" Actions
- Actions (VSports最新版本)
- "V体育ios版" Actions
- "VSports手机版" Actions
- Actions (V体育安卓版)
- V体育官网 - Actions
- "VSports" Actions
- "V体育官网入口" Actions
- "VSports在线直播" Actions
Substances
- "V体育官网入口" Actions
- VSports手机版 - Actions
- V体育官网入口 - Actions
- V体育ios版 - Actions
- "VSports手机版" Actions
- Actions (VSports最新版本)
"VSports手机版" Grants and funding
LinkOut - more resources
Full Text Sources
"V体育平台登录" Other Literature Sources
Molecular Biology Databases

