Cullin 1 functions as a centrosomal suppressor of centriole multiplication by regulating polo-like kinase 4 protein levels
- PMID: 19679553
- PMCID: PMC2727667
- DOI: 10.1158/0008-5472.CAN-09-1284
V体育安卓版 - Cullin 1 functions as a centrosomal suppressor of centriole multiplication by regulating polo-like kinase 4 protein levels
Abstract
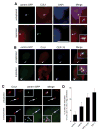
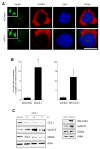
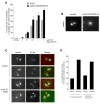
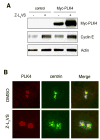
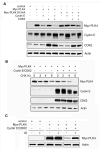
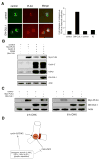
Abnormal centrosome and centriole numbers are frequently detected in tumor cells where they can contribute to mitotic aberrations that cause chromosome missegregation and aneuploidy. The molecular mechanisms of centriole overduplication in malignant cells, however, are poorly characterized VSports手机版. Here, we show that the core SKP1-cullin-F-box component cullin 1 (CUL1) localizes to maternal centrioles and that CUL1 is critical for suppressing centriole overduplication through multiplication, a recently discovered mechanism whereby multiple daughter centrioles form concurrently at single maternal centrioles. We found that this activity of CUL1 involves the degradation of Polo-like kinase 4 (PLK4) at maternal centrioles. PLK4 is required for centriole duplication and strongly stimulates centriole multiplication when aberrantly expressed. We found that CUL1 is critical for the degradation of active PLK4 following deregulation of cyclin E/cyclin-dependent kinase 2 activity, as is frequently observed in human cancer cells, as well as for baseline PLK4 protein stability. Collectively, our results suggest that CUL1 may function as a tumor suppressor by regulating PLK4 protein levels and thereby restraining excessive daughter centriole formation at maternal centrioles. .
Figures
References
-
- Azimzadeh J, Bornens M. Structure and duplication of the centrosome. J Cell Sci. 2007;120(Pt 13):2139–42. - PubMed
-
- Strnad P, Gonczy P. Mechanisms of procentriole formation. Trends Cell Biol. 2008;18(8):389–96. - PubMed
-
- Nigg EA. Centrosome aberrations: cause or consequence of cancer progression? Nature Rev Cancer. 2002;2:1–11. - PubMed
-
- Duensing S, Munger K. Centrosome abnormalities, genomic instability and carcinogenic progression. Biochim Biophys Acta. 2001;2(8):M81–8. - VSports app下载 - PubMed
-
- Fukasawa K. Oncogenes and tumour suppressors take on centrosomes. Nat Rev Cancer. 2007;7(12):911–24. - PubMed
Publication types
- Actions (V体育ios版)
- V体育官网入口 - Actions
MeSH terms
- V体育ios版 - Actions
- VSports在线直播 - Actions
- "VSports app下载" Actions
- VSports手机版 - Actions
- V体育官网 - Actions
- VSports在线直播 - Actions
- "V体育安卓版" Actions
- Actions (V体育安卓版)
- "V体育ios版" Actions
- "VSports注册入口" Actions
- Actions (VSports)
- V体育平台登录 - Actions
- V体育官网 - Actions
V体育2025版 - Substances
- Actions (V体育2025版)
- "V体育ios版" Actions
- VSports在线直播 - Actions
- Actions (VSports注册入口)
- "VSports" Actions
- Actions (V体育官网)
- V体育安卓版 - Actions
Grants and funding
"VSports最新版本" LinkOut - more resources
Full Text Sources
"V体育2025版" Other Literature Sources

