"V体育安卓版" Specific iron chelators determine the route of ferritin degradation
- PMID: 19671920
- PMCID: PMC2777130
- DOI: 10.1182/blood-2009-05-224188 (VSports注册入口)
Specific iron chelators determine the route of ferritin degradation
Abstract
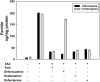
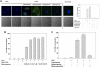
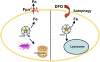
Deferoxamine (DFO) is a high-affinity Fe (III) chelator produced by Streptomyces pilosus. DFO is used clinically to remove iron from patients with iron overload disorders. Orally administered DFO cannot be absorbed, and therefore it must be injected. Here we show that DFO induces ferritin degradation in lysosomes through induction of autophagy. DFO-treated cells show cytosolic accumulation of LC3B, a critical protein involved in autophagosomal-lysosomal degradation. Treatment of cells with the oral iron chelators deferriprone and desferasirox did not show accumulation of LC3B, and degradation of ferritin occurred through the proteasome VSports手机版. Incubation of DFO-treated cells with 3-methyladenine, an autophagy inhibitor, resulted in degradation of ferritin by the proteasome. These results indicate that ferritin degradation occurs by 2 routes: a DFO-induced entry of ferritin into lysosomes and a cytosolic route in which iron is extracted from ferritin before degradation by the proteasome. .
Figures




Comment in
-
Mining ferritin iron: 2 pathways. (VSports)Blood. 2009 Nov 12;114(20):4325-6. doi: 10.1182/blood-2009-08-239913. Blood. 2009. PMID: 19965706 No abstract available.
"VSports" References
-
- Chasteen ND, Harrison PM. Mineralization in ferritin: an efficient means of iron storage. J Struct Biol. 1999;126(3):182–194. - PubMed
-
- Theil EC. Iron, ferritin, and nutrition. Annu Rev Nutr. 2004;24:327–343. - PubMed
-
- Rouault TA. The role of iron regulatory proteins in mammalian iron homeostasis and disease. Nat Chem Biol. 2006;2(8):406–414. - PubMed
-
- De Domenico I, Vaughn MB, Li L, et al. Ferroportin-mediated mobilization of ferritin iron precedes ferritin degradation by the proteasome. EMBO J. 2006;25(22):5396–5404. - "VSports在线直播" PMC - PubMed
-
- Bridges KR. Ascorbic acid inhibits lysosomal autophagy of ferritin. J Biol Chem. 1987;262(30):14773–14778. - PubMed
Publication types
MeSH terms
- V体育安卓版 - Actions
- Actions (VSports最新版本)
- Actions (V体育2025版)
- "VSports在线直播" Actions
- Actions (VSports最新版本)
- Actions (V体育官网入口)
V体育官网 - Substances
- "VSports注册入口" Actions
- Actions (VSports)
- Actions (V体育平台登录)
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

