"V体育官网" Pattern of expression and substrate specificity of chloroplast ferredoxins from Chlamydomonas reinhardtii
- PMID: 19586916
- PMCID: "VSports在线直播" PMC2757988
- DOI: 10.1074/jbc.M109.023622
Pattern of expression and substrate specificity of chloroplast ferredoxins from Chlamydomonas reinhardtii
Abstract
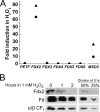
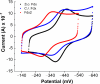
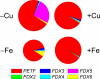
Ferredoxin (Fd) is the major iron-containing protein in photosynthetic organisms and is central to reductive metabolism in the chloroplast. The Chlamydomonas reinhardtii genome encodes six plant type [Fe2S2] ferredoxins, products of PETF, FDX2-FDX6. We performed the functional analysis of these ferredoxins by localizing Fd, Fdx2, Fdx3, and Fdx6 to the chloroplast by using isoform-specific antibodies and monitoring the pattern of gene expression by iron and copper nutrition, nitrogen source, and hydrogen peroxide stress. In addition, we also measured the midpoint redox potentials of Fd and Fdx2 and determined the kinetic parameters of their reactions with several ferredoxin-interacting proteins, namely nitrite reductase, Fd:NADP+ oxidoreductase, and Fd:thioredoxin reductase. We found that each of the FDX genes is differently regulated in response to changes in nutrient supply. Moreover, we show that Fdx2 (Em = -321 mV), whose expression is regulated by nitrate, is a more efficient electron donor to nitrite reductase relative to Fd. Overall, the results suggest that each ferredoxin isoform has substrate specificity and that the presence of multiple ferredoxin isoforms allows for the allocation of reducing power to specific metabolic pathways in the chloroplast under various growth conditions VSports手机版. .
"VSports手机版" Figures







References
-
- Fukuyama K. (2004) Photosynth. Res. 81, 289–301 - "V体育安卓版" PubMed
-
- Tagawa K., Arnon D. I. (1968) Biochim. Biophys. Acta 153, 602–613 - PubMed
-
- Mortenson L. E., Valentine R. C., Carnahan J. E. (1962) Biochem. Biophys. Res. Commun. 7, 448–452 - PubMed
-
- Lemaire S. D., Michelet L., Zaffagnini M., Massot V., Issakidis-Bourguet E. (2007) Curr. Genet. 51, 343–365 - PubMed
Publication types
- Actions (VSports注册入口)
MeSH terms
- "V体育安卓版" Actions
- "V体育安卓版" Actions
- VSports在线直播 - Actions
- "V体育官网" Actions
- Actions (V体育平台登录)
- Actions (VSports最新版本)
- "VSports app下载" Actions
- "VSports在线直播" Actions
- "VSports" Actions
- Actions (V体育官网)
- "V体育ios版" Actions
- V体育ios版 - Actions
- "VSports" Actions
- VSports最新版本 - Actions
- "V体育官网" Actions
- Actions (V体育官网)
V体育安卓版 - Substances
- VSports在线直播 - Actions
- "VSports" Actions
Associated data
- "V体育平台登录" Actions
- VSports - Actions
- "VSports在线直播" Actions
Grants and funding (VSports手机版)
LinkOut - more resources
Full Text Sources
"VSports注册入口" Other Literature Sources

