Inactivation of klotho function induces hyperphosphatemia even in presence of high serum fibroblast growth factor 23 levels in a genetically engineered hypophosphatemic (Hyp) mouse model
- PMID: 19584304
- PMCID: PMC2775000
- DOI: 10.1096/fj.08-123992
Inactivation of klotho function induces hyperphosphatemia even in presence of high serum fibroblast growth factor 23 levels in a genetically engineered hypophosphatemic (Hyp) mouse model
Abstract
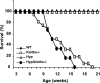
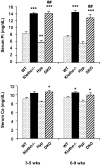
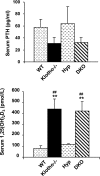
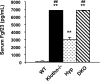
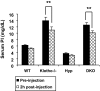
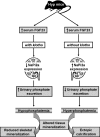
Hyp mice possess a mutation that inactivates the phosphate-regulating gene, which is homologous to the endopeptidases of the X-chromosome (PHEX). The mutation is associated with severe hypophosphatemia due to excessive urinary phosphate wasting. Such urinary phosphate wasting in Hyp mice is associated with an increased serum accumulation of fibroblast growth factor (FGF) 23. We wanted to determine the biological significance of increased serum FGF23 levels and concomitant hypophosphatemia in Hyp mice and to evaluate whether FGF23 activity could be modified by manipulating klotho (a cofactor of FGF23 signaling). We generated Hyp and klotho double-mutant mice (Hyp/klotho(-/-)). Severe hypophosphatemia of Hyp mice was reversed to hyperphosphatemia in Hyp/klotho(-/-) double mutants, despite the fact that the double mutants showed significantly increased serum levels of FGF23. Hyperphosphatemia in Hyp/klotho(-/-) mice was associated with increased renal expression of sodium/phosphate cotransporter 2a (NaPi2a) protein. Exogenous injection of bioactive parathyroid hormone 1-34 down-regulated renal expression of NaPi2a and consequently reduced serum levels of phosphate in Hyp/klotho(-/-) mice. Moreover, in contrast to the Hyp mice, the Hyp/klotho(-/-) mice showed significantly higher serum levels of 1,25-dihydroxyvitamin D and developed extensive calcification in soft tissues and vascular walls. Furthermore, compared with the Hyp mice, Hyp/klotho(-/-) mice were smaller in size, showed features of generalized tissue atrophy, and generally died by 15-20 wk of age. Our in vivo studies provide genetic evidence for a pathological role of increased FGF23 activities in regulating abnormal phosphate homeostasis in Hyp mice. Moreover, these results suggest that even when serum levels of FGF23 are significantly high, in the absence of klotho, FGF23 is unable to regulate systemic phosphate homeostasis VSports手机版. Our in vivo observations have significant clinical implications in diseases associated with increased FGF23 activity and suggest that the functions of FGF23 can be therapeutically modulated by manipulating the effects of klotho. .
Figures

References
-
- Yu X, White K E. FGF23 and disorders of phosphate homeostasis. Cytokine Growth Factor Rev. 2005;16:221–232. - PubMed
-
- Fukumoto S. Physiological regulation and disorders of phosphate metabolism—pivotal role of fibroblast growth factor 23. Intern Med. 2008;47:337–343. - PubMed
-
- Razzaque M S, St-Arnaud R, Taguchi T, Lanske B. FGF-23, vitamin D and calcification: the unholy triad. Nephrol Dial Transplant. 2005;20:2032–2035. - PubMed (VSports app下载)
-
- Gaasbeek A, Meinders A E. Hypophosphatemia: an update on its etiology and treatment. Am J Med. 2005;118:1094–1101. - PubMed
Publication types
"V体育ios版" MeSH terms
- "VSports最新版本" Actions
- "VSports注册入口" Actions
- Actions (V体育安卓版)
- "VSports" Actions
- "VSports手机版" Actions
- "V体育官网入口" Actions
- "V体育平台登录" Actions
- V体育安卓版 - Actions
- Actions (V体育2025版)
Substances
- "VSports手机版" Actions
- "V体育官网" Actions
- Actions (VSports手机版)
- VSports - Actions
"VSports app下载" Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

