Combination of taxol and Bcl-2 siRNA induces apoptosis in human glioblastoma cells and inhibits invasion, angiogenesis and tumour growth
- PMID: 19473291
- PMCID: PMC4496127
- DOI: 10.1111/j.1582-4934.2008.00539.x
Combination of taxol and Bcl-2 siRNA induces apoptosis in human glioblastoma cells and inhibits invasion, angiogenesis and tumour growth (V体育2025版)
Abstract
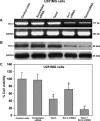
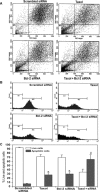
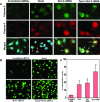
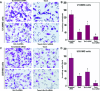
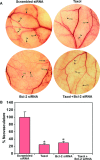
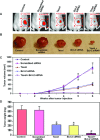
Taxol is a powerful chemotherapeutic agent that binds to microtubules to prevent tumour cell division. However, a traditional high dose of taxol may also induce apoptosis in normal cells. The anti-apoptotic molecule Bcl-2 is up-regulated in tumour cells to prevent apoptosis. We designed this study to determine whether use of a low dose of taxol and anti-apoptotic Bcl-2 gene silencing would effectively induce apoptosis in human glioblastoma U251MG cells and also inhibit invasion, angiogenesis and intracranial as well as subcutaneous tumour growth. We treated the cells with either 100 nM taxol or transfected with a plasmid vector expressing Bcl-2 siRNA or both agents together for 72 h. Knockdown of Bcl-2 potentiated efficacy of taxol for cell death. Fluorescence-activated cell sorting analysis, double immunofluorescent staining and TUNEL assay demonstrated apoptosis in about 70% of the cells after treatment with the combination of taxol and Bcl-2 siRNA. In vitro Matrigel invasion assay demonstrated dramatic decrease in glioblastoma cell invasion and in vivo angiogenesis assay showed complete inhibition of neovascularization in athymic nude mice after treatment with the combination. Further, treatment with the combination of taxol and Bcl-2 siRNA caused suppression of intracranial tumour growth and subcutaneous solid tumour development. In conclusion, our results indicate that the combination of taxol and Bcl-2 siRNA effectively induces apoptosis and inhibits glioblastoma cell invasion, angiogenesis and intracranial as well as subcutaneous tumour growth. Therefore, the combination of a low dose of taxol and Bcl-2 siRNA is a promising therapeutic strategy for controlling the aggressive growth of human glioblastoma VSports手机版. .
Figures

References (VSports在线直播)
-
- Pulkkanen KJ, Yla-Herttuala S. Gene therapy for malignant glioma: current clinical status. Mol Ther. 2005;12:585–98. - PubMed
-
- Donaldson SS, Laningham F, Fisher PG. Advances toward an understanding of brainstem gliomas. J Clin Oncol. 2006;24:1266–72. - PubMed
-
- Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Cancer. 2002;2:91–100. - PubMed (VSports最新版本)
-
- Zagzag D, Friedlander DR, Margolis B, et al. Molecular events implicated in brain tumor angiogenesis and invasion. Pediatr Neurosurg. 2000;33:49–55. - PubMed
-
- Kitada S, Pedersen IM, Schimmer AD, et al. Dysregulation of apoptosis genes in hematopoietic malignancies. Oncogene. 2002;21:3459–74. - PubMed
Publication types
- Actions (V体育官网入口)
MeSH terms
- VSports app下载 - Actions
- V体育平台登录 - Actions
- VSports最新版本 - Actions
- "VSports在线直播" Actions
- V体育官网 - Actions
- Actions (V体育平台登录)
- "VSports注册入口" Actions
- VSports注册入口 - Actions
- Actions (V体育安卓版)
- VSports注册入口 - Actions
- "VSports在线直播" Actions
- "V体育安卓版" Actions
- "V体育官网" Actions
"VSports在线直播" Substances
- Actions (VSports注册入口)
Grants and funding
"V体育平台登录" LinkOut - more resources
Full Text Sources

