Longitudinal assessment of morbidity and acute graft-versus-host disease after allogeneic hematopoietic cell transplantation: retrospective analysis of a multicenter phase III study
- PMID: 19450760
- PMCID: PMC2684865
- DOI: V体育平台登录 - 10.1016/j.bbmt.2009.03.009
Longitudinal assessment of morbidity and acute graft-versus-host disease after allogeneic hematopoietic cell transplantation: retrospective analysis of a multicenter phase III study
Abstract
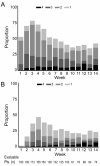
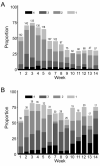
Because morbidity early after hematopoietic cell transplantation (HCT) results in large part from the development of acute graft-versus-host disease (GVHD), we previously proposed that a longitudinal assessment of morbidity involving the skin, liver, and gastrointestinal (GI) tract might provide a more complete, objective approach for comparing 2 arms of open-label randomized clinical trials for acute GVHD prevention. In this study, we determined both morbidity across time and GVHD across time in a retrospective analysis of a database from an open-label randomized clinical trial comparing tacrolimus/methotrexate and cyclosporine/methotrexate after myeloablative conditioning and marrow transplantation from HLA-matched unrelated donors VSports手机版. The results confirm differences in overall morbidity across time in patients with peak grade II-IV GVHD compared with those with grade 0-I GVHD, but no significant differences in morbidity associated with grade II GVHD compared with grade 0-I GVHD. We observed less skin morbidity and a trend toward less liver morbidity across time in the tacrolimus group (P = . 04 and . 09, respectively), but not for GI morbidity or overall morbidity, despite significantly decreased skin and liver stages and overall grades of GVHD across time in this group. Thus, our objective assessment of differences in morbidity (regardless of cause) as a measure of acute GVHD in a randomized clinical trial of acute GVHD prevention has only limited utility. The difficulty of demonstrating clinical benefits from objective parameters, such as survival and morbidity, and the subjectivity of grading acute GVHD emphasize the need for blinded assessments in clinical trials of GVHD prevention. .
Figures
References
-
- Sullivan KM. Graft-vs.-host disease. In: Blume KG, Forman SJ, Appelbaum FR, editors. Thomas' Hematopoietic Cell Transplantation. Blackwell Publishing Ltd; Oxford, UK: 2004. pp. 635–664.
-
- Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of Rgraft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304. - PubMed
-
- Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825–828. - PubMed
-
- Thomas ED, Storb R, Clift RA, et al. Bone-marrow transplantation. N Engl J Med. 1975;292:832–843. 895–902. - "V体育平台登录" PubMed
-
- Rowlings PA, Przepiorka D, Klein JP, et al. IBMTR Severity Index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. Br J Haematol. 1997;97:855–864. - PubMed
Publication types
- VSports最新版本 - Actions
MeSH terms
- VSports app下载 - Actions
- "VSports app下载" Actions
- "VSports注册入口" Actions
- VSports注册入口 - Actions
- Actions (VSports最新版本)
- Actions (VSports最新版本)
- Actions (VSports)
- "V体育官网入口" Actions
- "V体育2025版" Actions
- Actions (V体育2025版)
- "VSports在线直播" Actions
Substances (VSports注册入口)
- "VSports注册入口" Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

