"VSports在线直播" Central memory CD8+ T cells induce graft-versus-host disease and mediate graft-versus-leukemia
- PMID: 19414745
- PMCID: "V体育ios版" PMC9844260
- DOI: 10.4049/jimmunol.0802212
Central memory CD8+ T cells induce graft-versus-host disease and mediate graft-versus-leukemia
Abstract
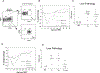
In allogeneic hemopoietic stem cell transplantation, mature donor alphabeta T cells in the allograft promote T cell reconstitution in the recipient and mediate the graft-vs-leukemia (GVL) effect. Unfortunately, donor T cells can attack nonmalignant host tissues and cause graft-vs-host disease (GVHD). It has previously been shown that effector memory T cells not primed to alloantigen do not cause GVHD yet transfer functional T cell memory and mediate GVL. Recently, central memory T cells (T(CM)) have also been reported to not cause GVHD. In contrast, in this study, we demonstrate that purified CD8(+) T(CM) not specifically primed to alloantigens mediate GVHD in the MHC-mismatched C57BL/6 (B6)-->BALB/c and the MHC-matched, multiple minor histocompatibility Ag-mismatched C3H. SW-->B6 strain pairings. CD8(+) T(CM) and naive T cells (T(N)) caused similar histological disease in liver, skin, and bowel VSports手机版. B6 CD8(+) T(CM) and T(N) similarly expanded in BALB/c recipients, and the majority of their progeny produced IFN-gamma upon restimulation. However, in both models, CD8(+) T(CM) induced milder clinical GVHD than did CD8(+) T(N). Nonetheless, CD8(+) T(CM) and T(N) were similarly potent mediators of GVL against a mouse model of chronic-phase chronic myelogenous leukemia. Thus, in contrast to what was previously thought, CD8(+) T(CM) are capable of inducing GVHD and are substantially different from T(EM) but only subtly so from T(N). .
Conflict of interest statement
Disclosures
The authors have no conflicting financial interests.
Figures (V体育官网)




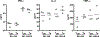

References
-
- Mackall CL, and Gress RE 1997. Pathways of T-cell regeneration in mice and humans: implications for bone marrow transplantation and immunotherapy. Immunol Rev 157:61–72. - PubMed
-
- Collins RH Jr., Shpilberg O, Drobyski WR, Porter DL, Giralt S, Champlin R, Goodman SA, Wolff SN, Hu W, Verfaillie C, List A, Dalton W, Ognoskie N, Chetrit A, Antin JH, and Nemunaitis J 1997. Donor leukocyte infusions in 140 patients with relapsed malignancy after allogeneic bone marrow transplantation. J Clin Oncol 15:433–444. - PubMed (V体育2025版)
-
- Kolb HJ, Schattenberg A, Goldman JM, Hertenstein B, Jacobsen N, Arcese W, Ljungman P, Ferrant A, Verdonck L, Niederwieser D, and et al. 1995. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. European Group for Blood and Marrow Transplantation Working Party Chronic Leukemia. Blood 86:2041–2050. - "V体育官网入口" PubMed
-
- Porter DL, Collins RH Jr., Shpilberg O, Drobyski WR, Connors JM, Sproles A, and Antin JH 1999. Long-term follow-up of patients who achieved complete remission after donor leukocyte infusions. Biol Blood Marrow Transplant 5:253–261. - V体育安卓版 - PubMed
-
- Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ, Rimm AA, Ringden O, Rozman C, Speck B, and et al. 1990. Graft-versus-leukemia reactions after bone marrow transplantation. Blood 75:555–562. - "V体育安卓版" PubMed
V体育2025版 - Publication types
- "V体育官网" Actions
- Actions (VSports app下载)
MeSH terms
- VSports手机版 - Actions
- Actions (V体育官网入口)
- "V体育ios版" Actions
- V体育ios版 - Actions
- "VSports在线直播" Actions
- "V体育2025版" Actions
- Actions (V体育ios版)
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

