Nitric oxide-mediated intestinal injury is required for alcohol-induced gut leakiness and liver damage
- PMID: 19389191
- PMCID: PMC2950098
- DOI: 10.1111/j.1530-0277.2009.00946.x
Nitric oxide-mediated intestinal injury is required for alcohol-induced gut leakiness and liver damage (VSports最新版本)
Abstract
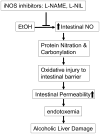
Background: Alcoholic liver disease (ALD) requires endotoxemia and is commonly associated with intestinal barrier leakiness. Using monolayers of intestinal epithelial cells as an in vitro barrier model, we showed that ethanol-induced intestinal barrier disruption is mediated by inducible nitric oxide synthase (iNOS) upregulation, nitric oxide (NO) overproduction, and oxidation/nitration of cytoskeletal proteins. We hypothesized that iNOS inhibitors [NG-nitro-l-arginine methyl ester (l-NAME), l-N(6)-(1-iminoethyl)-lysine (l-NIL)] in vivo will inhibit the above cascade and liver injury in an animal model of alcoholic steatohepatitis (ASH). VSports手机版.
Methods: Male Sprague-Dawley rats were gavaged daily with alcohol (6 g/kg/d) or dextrose for 10 weeks +/- l-NAME, l-NIL, or vehicle. Systemic and intestinal NO levels were measured by nitrites and nitrates in urine and tissue samples, oxidative damage to the intestinal mucosa by protein carbonyl and nitrotyrosine, intestinal permeability by urinary sugar tests, and liver injury by histological inflammation scores, liver fat, and myeloperoxidase activity. V体育安卓版.
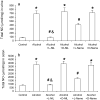
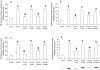
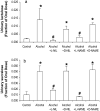
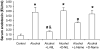
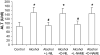
Results: Alcohol caused tissue oxidation, gut leakiness, endotoxemia, and ASH. l-NIL and l-NAME, but not the d-enantiomers, attenuated all steps in the alcohol-induced cascade including NO overproduction, oxidative tissue damage, gut leakiness, endotoxemia, hepatic inflammation, and liver injury. V体育ios版.
Conclusions: The mechanism we reported for alcohol-induced intestinal barrier disruption in vitro - NO overproduction, oxidative tissue damage, leaky gut, endotoxemia, and liver injury - appears to be relevant in vivo in an animal model of alcohol-induced liver injury. That iNOS inhibitors attenuated all steps of this cascade suggests that prevention of this cascade in alcoholics will protect the liver against the injurious effects of chronic alcohol and that iNOS may be a useful target for prevention of ALD. VSports最新版本.
Figures


References
-
- Alican I, Kubes P. A critical role for nitric oxide in intestinal barrier function and dysfunction. Am J Physiol. 1996;270(2 Pt 1):G225–37. - PubMed
-
- Aram G, Potter JJ, Liu X, Torbenson MS, Mezey E. Lack of inducible nitric oxide synthase leads to increased hepatic apoptosis and decreased fibrosis in mice after chronic carbon tetrachloride administration. Hepatology. 2008;47(6):2051–8. - "V体育官网" PubMed
-
- Banan A, Choudhary S, Zhang Y, Fields JZ, Keshavarzian A. Ethanol-induced barrier dysfunction and its prevention by growth factors in human intestinal monolayers: evidence for oxidative and cytoskeletal mechanisms. J Pharmacol Exp Ther. 1999;291(3):1075–85. - PubMed
-
- Banan A, Fields JZ, Decker H, Zhang Y, Keshavarzian A. Nitric oxide and its metabolites mediate ethanol-induced microtubule disruption and intestinal barrier dysfunction. J Pharmacol Exp Ther. 2000;294(3):997–1008. - PubMed
-
- Banan A, Fields JZ, Zhang Y, Keshavarzian A. iNOS upregulation mediates oxidant-induced disruption of F-actin and barrier of intestinal monolayers. Am J Physiol Gastrointest Liver Physiol. 2001;280(6):G1234–46. - "VSports注册入口" PubMed
Publication types
MeSH terms
- Actions (VSports手机版)
- Actions (V体育ios版)
- "VSports注册入口" Actions
- "V体育2025版" Actions
- V体育平台登录 - Actions
- Actions (VSports手机版)
- V体育平台登录 - Actions
Substances
- "VSports注册入口" Actions
- Actions (VSports注册入口)
- Actions (V体育官网入口)
Grants and funding (V体育官网)
LinkOut - more resources
Full Text Sources
VSports - Other Literature Sources
Research Materials
Miscellaneous

