Normally occurring NKG2D+CD4+ T cells are immunosuppressive and inversely correlated with disease activity in juvenile-onset lupus
- PMID: 19289577
- PMCID: VSports - PMC2715116
- DOI: 10.1084/jem.20081648
Normally occurring NKG2D+CD4+ T cells are immunosuppressive and inversely correlated with disease activity in juvenile-onset lupus (V体育官网)
"VSports最新版本" Abstract
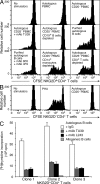
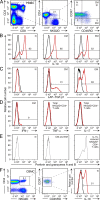
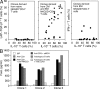
The NKG2D receptor stimulates natural killer cell and T cell responses upon engagement of ligands associated with malignancies and certain autoimmune diseases. However, conditions of persistent NKG2D ligand expression can lead to immunosuppression. In cancer patients, tumor expression and shedding of the MHC class I-related chain A (MICA) ligand of NKG2D drives proliferative expansions of NKG2D(+)CD4(+) T cells that produce interleukin-10 (IL-10) and transforming growth factor-beta, as well as Fas ligand, which inhibits bystander T cell proliferation in vitro. Here, we show that increased frequencies of functionally equivalent NKG2D(+)CD4(+) T cells are inversely correlated with disease activity in juvenile-onset systemic lupus erythematosus (SLE), suggesting that these T cells may have regulatory effects VSports手机版. The NKG2D(+)CD4(+) T cells correspond to a normally occurring small CD4 T cell subset that is autoreactive, primed to produce IL-10, and clearly distinct from proinflammatory and cytolytic CD4 T cells with cytokine-induced NKG2D expression that occur in rheumatoid arthritis and Crohn's disease. As classical regulatory T cell functions are typically impaired in SLE, it may be clinically significant that the immunosuppressive NKG2D(+)CD4(+) T cells appear functionally uncompromised in this disease. .
Figures





References (V体育安卓版)
-
- Long E.O. 1999. Regulation of immune responses through inhibitory receptors.Annu. Rev. Immunol. 17:875–904 - PubMed
-
- Huard B., Karlsson L. 2000. KIR expression on self-reactive CD8+ T cells is controlled by T-cell receptor engagement.Nature. 403:325–328 - "V体育平台登录" PubMed
-
- Moser J.M., Gibbs J., Jensen P.E., Lukacher A.E. 2002. CD94-NKG2A receptors regulate antiviral CD8(+) T cell responses.Nat. Immunol. 3:189–195 - PubMed
-
- Lanier L.L. 2008. Up on the tightrope: natural killer cell activation and inhibition.Nat. Immunol. 9:495–502 - "V体育ios版" PMC - PubMed
-
- Groh V., Steinle A., Bauer S., Spies T. 1998. Recognition of stress-induced MHC molecules by intestinal epithelial gammadelta T cells.Science. 279:1737–1740 - PubMed
VSports注册入口 - Publication types
- VSports最新版本 - Actions
- "V体育官网" Actions
MeSH terms
- "VSports" Actions
- Actions (V体育2025版)
- "VSports" Actions
- V体育安卓版 - Actions
- "VSports最新版本" Actions
- VSports最新版本 - Actions
- V体育官网 - Actions
- Actions (VSports)
V体育2025版 - Substances
- V体育官网入口 - Actions
- Actions (VSports在线直播)
- Actions (VSports app下载)
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

