An Ehrlichia chaffeensis tandem repeat protein interacts with multiple host targets involved in cell signaling, transcriptional regulation, and vesicle trafficking
- PMID: 19273555
- PMCID: "VSports" PMC2681728
- DOI: "V体育安卓版" 10.1128/IAI.00027-09
"V体育官网入口" An Ehrlichia chaffeensis tandem repeat protein interacts with multiple host targets involved in cell signaling, transcriptional regulation, and vesicle trafficking
Abstract
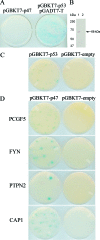
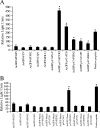
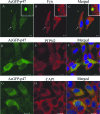
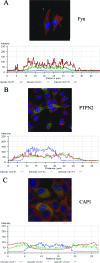
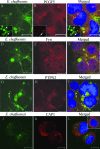
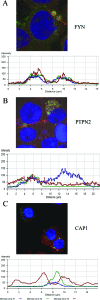
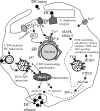
Ehrlichia chaffeensis is an obligately intracellular bacterium that exhibits tropism for mononuclear phagocytes forming cytoplasmic membrane-bound microcolonies called morulae. To survive and replicate within phagocytes, E. chaffeensis exploits the host cell by modulating a number of host cell processes, but the ehrlichial effector proteins involved are unknown. In this study, we determined that p47, a secreted, differentially expressed, tandem repeat (TR) protein, interacts with multiple host proteins associated with cell signaling, transcriptional regulation, and vesicle trafficking. Yeast two-hybrid analysis revealed that p47 interacts with polycomb group ring finger 5 (PCGF5) protein, Src protein tyrosine kinase FYN (FYN), protein tyrosine phosphatase non-receptor type 2 (PTPN2), and adenylate cyclase-associated protein 1 (CAP1). p47 interaction with these proteins was further confirmed by coimmunoprecipitation assays and colocalization in HeLa cells transfected with p47-green fluorescent fusion protein (AcGFP1-p47). Moreover, confocal microscopy demonstrated p47-expressing dense-cored (DC) ehrlichiae colocalized with PCGF5, FYN, PTPN2, and CAP1. An amino-terminally truncated form of p47 containing TRs interacted only with PCGF5 and not with FYN, PTPN2, and CAP1, indicating differences in p47 domains that are involved in these interactions VSports手机版. These results demonstrate that p47 is involved in a complex network of interactions involving numerous host cell proteins. Furthermore, this study provides a new insight into the molecular and functional distinction of DC ehrlichiae, as well as the effector proteins involved in facilitating ehrlichial survival in mononuclear phagocytes. .
Figures

References
-
- Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 253389-3402. - PMC (VSports app下载) - PubMed
-
- Anderson, R. G. 1998. The caveolae membrane system. Annu. Rev. Biochem. 67199-225. - PubMed
-
- Barnewall, R. E., N. Ohashi, and Y. Rikihisa. 1999. Ehrlichia chaffeensis and E. sennetsu, but not the human granulocytic ehrlichiosis agent, colocalize with transferrin receptor and up-regulate transferrin receptor mRNA by activating iron-responsive protein 1. Infect. Immun. 672258-2265. - PMC - PubMed
-
- Bertling, E., O. Quintero-Monzon, P. K. Mattila, B. L. Goode, and P. Lappalainen. 2007. Mechanism and biological role of profilin-Srv2/CAP interaction. J. Cell Sci. 1201225-1234. - PubMed
Publication types (V体育平台登录)
V体育2025版 - MeSH terms
- Actions (VSports手机版)
- Actions (VSports app下载)
- V体育官网 - Actions
- "V体育ios版" Actions
- Actions (V体育安卓版)
- "V体育安卓版" Actions
- "VSports app下载" Actions
- "VSports在线直播" Actions
Substances
- V体育官网入口 - Actions
- Actions (V体育平台登录)
- "V体育ios版" Actions
- V体育2025版 - Actions
- V体育官网入口 - Actions
- "V体育官网" Actions
- "V体育官网" Actions
Grants and funding
"VSports手机版" LinkOut - more resources
Full Text Sources
VSports手机版 - Other Literature Sources
"VSports app下载" Molecular Biology Databases
V体育官网入口 - Research Materials
Miscellaneous

