Overexpression of the cellular DEK protein promotes epithelial transformation in vitro and in vivo
- PMID: 19223548
- PMCID: PMC2650744
- DOI: 10.1158/0008-5472.CAN-08-2304 (VSports注册入口)
Overexpression of the cellular DEK protein promotes epithelial transformation in vitro and in vivo
Abstract
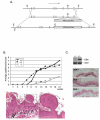
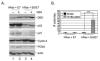
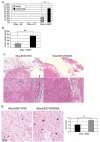
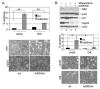
High levels of expression of the human DEK gene have been correlated with numerous human malignancies. Intracellular DEK functions have been described in vitro and include DNA supercoiling, DNA replication, RNA splicing, and transcription. We have shown that DEK also suppresses cellular senescence, apoptosis, and differentiation, thus promoting cell growth and survival in monolayer and organotypic epithelial raft models VSports手机版. Such functions are likely to contribute to cancer, but direct evidence to implicate DEK as an oncogene has remained elusive. Here, we show that in line with an early role in tumorigenesis, murine papilloma formation in a classical chemical carcinogenesis model was reduced in DEK knockout mice. Additionally, human papillomavirus E6/E7, hRas, and DEK cooperated in the transformation of keratinocytes in soft agar and xenograft establishment, thus also implicating DEK in tumor promotion at later stages. Finally, adenoviral DEK depletion via short hairpin RNA expression resulted in cell death in human tumor cells in vitro and in vivo, but did not significantly affect differentiated epithelial cells. Taken together, our data uncover oncogenic DEK activities as postulated from its frequent up-regulation in human malignancies, and suggest that the targeted suppression of DEK may become a strategic approach to the treatment of cancer. .
Figures

References
-
- Wu Q, Hoffmann MJ, Hartmann FH, Schulz WA. Amplification and overexpression of the ID4 gene at 6p22.3 in bladder cancer. Mol Cancer. 2005;4(1):16. - V体育安卓版 - PMC - PubMed
-
- Kroes RA, Jastrow A, McLone MG, et al. The identification of novel therapeutic targets for the treatment of malignant brain tumors. Cancer Lett. 2000;156(2):191–8. - VSports在线直播 - PubMed
-
- Carro MS, Spiga FM, Quarto M, et al. DEK Expression is controlled by E2F and deregulated in diverse tumor types. Cell Cycle. 2006;5(11):1202–7. - PubMed
V体育2025版 - Publication types
"V体育安卓版" MeSH terms
- Actions (V体育安卓版)
- "VSports app下载" Actions
- Actions (V体育官网)
- "V体育2025版" Actions
- "VSports最新版本" Actions
- Actions (V体育2025版)
- VSports app下载 - Actions
- VSports最新版本 - Actions
- VSports最新版本 - Actions
- V体育官网入口 - Actions
Substances
- "VSports注册入口" Actions
- VSports最新版本 - Actions
- "V体育官网入口" Actions
- "V体育官网" Actions
- V体育ios版 - Actions
- V体育安卓版 - Actions
- "V体育ios版" Actions
- "VSports手机版" Actions
- Actions (VSports最新版本)
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
"V体育官网入口" Miscellaneous

