Regulation of neuronal cell death by MST1-FOXO1 signaling
- PMID: 19221179
- PMCID: "VSports app下载" PMC2670133
- DOI: 10.1074/jbc.M900461200
"VSports注册入口" Regulation of neuronal cell death by MST1-FOXO1 signaling
"V体育官网" Abstract
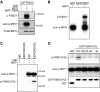
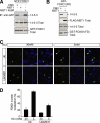
The protein kinase mammalian Sterile 20-like kinase 1 (MST1) plays a critical role in the regulation of cell death VSports手机版. Recent studies suggest that MST1 mediates oxidative stress-induced neuronal cell death by phosphorylating the transcription factor FOXO3 at serine 207, a site that is conserved in other FOXO family members. Here, we show that MST1-induced phosphorylation of FOXO1 at serine 212, corresponding to serine 207 in FOXO3, disrupts the association of FOXO1 with 14-3-3 proteins. Accordingly, MST1 mediates the nuclear translocation of FOXO1 in primary rat cerebellar granule neurons that are deprived of neuronal activity. We also find a requirement for MST1 in cell death of granule neurons upon withdrawal of growth factors and neuronal activity, and MST1 induces cell death in a FOXO1-dependent manner. Finally, we show that the MST1-regulatory, scaffold protein Nore1 is required for survival factor deprivation induced neuronal death. Collectively, these findings define MST1-FOXO1 signaling as an important link survival factor deprivation-induced neuronal cell death with implications for our understanding of brain development and neurological diseases. .
Figures

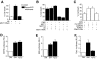
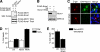
References
-
- Oppenheim, R. (1989) Annu. Rev. Neurosci. 14, 453-501 - PubMed
-
- Pettmann, B., and Henderson, C. E. (1998) Neuron 20, 633-647 - PubMed
-
- Yuan, J., and Yankner, B. A. (2000) Nature 407, 802-809 - PubMed
-
- Mattson, M. P. (2000) Nat. Rev. Mol. Cell Biol. 1, 120-129 - V体育安卓版 - PubMed
-
- Choi, D. W. (1996) Curr. Opin. Neurobiol. 6, 667-672 - PubMed
Publication types
- Actions (VSports在线直播)
- "V体育官网" Actions
MeSH terms
- Actions (VSports手机版)
- Actions (VSports最新版本)
- "V体育2025版" Actions
- VSports最新版本 - Actions
- Actions (VSports在线直播)
- V体育ios版 - Actions
- V体育平台登录 - Actions
- "V体育安卓版" Actions
- V体育官网 - Actions
- "V体育官网入口" Actions
- Actions (V体育2025版)
Substances
- Actions (VSports app下载)
- "VSports注册入口" Actions
- Actions (VSports最新版本)
- "V体育平台登录" Actions
"V体育安卓版" Grants and funding
"VSports最新版本" LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

