The P2X(7) receptor-pannexin connection to dye uptake and IL-1beta release
- PMID: 19212823
- PMCID: PMC2686830
- DOI: 10.1007/s11302-009-9141-7
The P2X(7) receptor-pannexin connection to dye uptake and IL-1beta release
Abstract
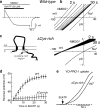
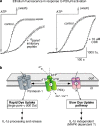
The P2X(7) receptor (P2X(7)R) is uniquely associated with two distinct cellular responses: activation of a dye-permeable pathway allowing passage of molecules up to 900 Da and rapid release of the pro-inflammatory cytokine, interleukin-1beta (IL-1beta), from activated macrophage VSports手机版. How this dye uptake path forms and whether it is involved in IL-1beta release has not been known. Pannexin-1 is a recently identified protein found to physically associate with the P2X(7)R. Inhibition of pannexin-1 does not alter P2X(7)R ion channel activation or associated calcium flux but blocks one component of P2X(7)R-induced dye uptake and unmasks a slower, previously undetected, dye uptake pathway. Inhibition of pannexin-1 blocks P2X(7)R-mediated IL-1beta release from macrophage as well as release mediated by other stimuli which couple to activation of capase-1 and additionally inhibits the release of interleukin-1alpha, a member of the IL-1 family whose processing does not require caspase-1 activation. Thus, pannexin-1 is linked to both dye uptake and IL-1beta release but via distinct mechanisms. .
Figures

References
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1016/0006-291X(75)90207-7', 'is_inner': False, 'url': '"V体育2025版" https://doi.org/10.1016/0006-291x(75)90207-7'}, {'type': 'PubMed', 'value': '1039', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/1039/'}]}
- Rozengurt E, Heppel LA (1975) A specific effect of ATP on the permeability of transformed 3T3 cells. Biochem Biophys Res Commun 67:1581–1588 - PubMed (V体育平台登录)
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1038/279541a0', 'is_inner': False, 'url': 'https://doi.org/10.1038/279541a0'}, {'type': 'PubMed', 'value': '450099', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/450099/'}]}
- Cockcroft S, Gomperts BD (1979) ATP induces nucleotide permeability in rat mast cells. Nature 279:541–542 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1073/pnas.85.21.7988', 'is_inner': False, 'url': 'https://doi.org/10.1073/pnas.85.21.7988'}, {'type': 'PMC', 'value': 'PMC282339', 'is_inner': False, 'url': 'https://pmc.ncbi.nlm.nih.gov/articles/PMC282339/'}, {'type': 'PubMed', 'value': '3186701', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/3186701/'}]}
- Buisman HP, Steinberg TH, Fischbarg J, Silverstein SC, Vogelzang SA, Ince C, Ypey DL, Leijh PCJ (1988) Extracellular ATP induces a large nonselective conductance in macrophage plasma membranes. Proc Natl Acad Sci U S A 85:7988–7992 - PMC - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '2932438', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/2932438/' (VSports手机版)}]}
- Sung SS, Young JD, Origlio AM, Heiple JM, Kaback HR, Silverstein SC (1985) Extracellular ATP perturbs transmembrane ion fluxes, elevates cytosolic [Ca2+] and inhibits phagocytosis in mouse macrophages. J Biol Chem 260:13442–13449 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '2950094', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/2950094/'}]}
- Steinberg TH, Silverstein SC (1987) Extracellular ATP4− promotes cation fluxes in the J774 mouse macrophage cell line. J Biol Chem 262:3118–3122 - PubMed
LinkOut - more resources
V体育ios版 - Full Text Sources
Other Literature Sources
VSports在线直播 - Molecular Biology Databases

