Disruption of the Toxoplasma gondii parasitophorous vacuole by IFNgamma-inducible immunity-related GTPases (IRG proteins) triggers necrotic cell death
- PMID: 19197351
- PMCID: "VSports最新版本" PMC2629126
- DOI: "V体育安卓版" 10.1371/journal.ppat.1000288
Disruption of the Toxoplasma gondii parasitophorous vacuole by IFNgamma-inducible immunity-related GTPases (IRG proteins) triggers necrotic cell death
V体育官网入口 - Abstract
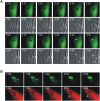
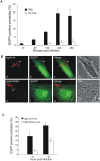
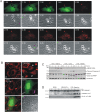
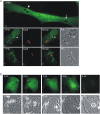
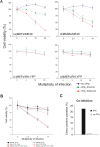
Toxoplasma gondii is a natural intracellular protozoal pathogen of mice and other small mammals VSports手机版. After infection, the parasite replicates freely in many cell types (tachyzoite stage) before undergoing a phase transition and encysting in brain and muscle (bradyzoite stage). In the mouse, early immune resistance to the tachyzoite stage is mediated by the family of interferon-inducible immunity-related GTPases (IRG proteins), but little is known of the nature of this resistance. We reported earlier that IRG proteins accumulate on intracellular vacuoles containing the pathogen, and that the vacuolar membrane subsequently ruptures. In this report, live-cell imaging microscopy has been used to follow this process and its consequences in real time. We show that the rupture of the vacuole is inevitably followed by death of the intracellular parasite, shown by its permeability to cytosolic protein markers. Death of the parasite is followed by the death of the infected cell. The death of the cell has features of pyronecrosis, including membrane permeabilisation and release of the inflammatory protein, HMGB1, but caspase-1 cleavage is not detected. This sequence of events occurs on a large scale only following infection of IFNgamma-induced cells with an avirulent strain of T. gondii, and is reduced by expression of a dominant negative mutant IRG protein. Cells infected by virulent strains rarely undergo necrosis. We did not find autophagy to play any role in the key steps leading to the death of the parasite. We conclude that IRG proteins resist infection by avirulent T. gondii by a novel mechanism involving disruption of the vacuolar membrane, which in turn ultimately leads to the necrotic death of the infected cell. .
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References (VSports app下载)
-
- Saeij JP, Boyle JP, Boothroyd JC. Differences among the three major strains of Toxoplasma gondii and their specific interactions with the infected host. Trends Parasitol. 2005;21:476–481. - PubMed
-
- Sibley LD, Boothroyd JC. Virulent strains of Toxoplasma gondii comprise a single clonal lineage. Nature. 1992;359:82–85. - VSports手机版 - PubMed
-
- Frenkel JK. Pathophysiology of toxoplasmosis. Parasitol Today. 1988;4:273–278. - PubMed (VSports在线直播)
-
- Hunter CA, Remington JS. Immunopathogenesis of toxoplasmic encephalitis. J Infect Dis. 1994;170:1057–1067. - "V体育2025版" PubMed
-
- Yap GS, Sher A. Cell-mediated immunity to Toxoplasma gondii: initiation, regulation and effector function. Immunobiology. 1999;201:240–247. - VSports最新版本 - PubMed
"V体育2025版" Publication types
"V体育平台登录" MeSH terms
- "VSports最新版本" Actions
- "VSports app下载" Actions
- Actions (V体育平台登录)
- "VSports最新版本" Actions
- V体育ios版 - Actions
- Actions (VSports注册入口)
- VSports app下载 - Actions
- Actions (V体育官网入口)
Substances
- "VSports注册入口" Actions
LinkOut - more resources
Full Text Sources

