Structural and functional analysis of a C3b-specific antibody that selectively inhibits the alternative pathway of complement
- PMID: 19196712
- PMCID: PMC2667734
- DOI: 10.1074/jbc.M809106200
Structural and functional analysis of a C3b-specific antibody that selectively inhibits the alternative pathway of complement
V体育平台登录 - Abstract
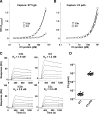
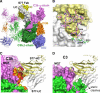
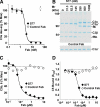
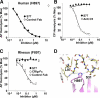
Amplification of the complement cascade through the alternative pathway can lead to excessive inflammation. Targeting C3b, a component central to the alternative pathway of complement, provides a powerful approach to inhibit complement-mediated immune responses and tissue injury. In the present study, phage display technology was employed to generate an antibody that selectively recognizes C3b but not the non-activated molecule C3 VSports手机版. The crystal structure of C3b in complex with a Fab fragment of this antibody (S77) illustrates the structural basis for this selectivity. Cleavage of C3 to C3b results in a plethora of structural changes within C3, including the rearrangement of macroglobulin domain 6 enabling binding of S77 to the adjacent macroglobulin domain 7 domain. S77 blocks binding of factor B to C3b inhibiting the first step in the formation of the alternative pathway C3 convertase. In addition, S77 inhibits C5 binding to C3b. This results in significantly reduced formations of anaphylatoxins and membrane-attack complexes. This study for the first time demonstrates the structural basis for complement inhibition by a C3b-selective antibody and provides insights into the molecular mechanisms of alternative pathway complement activation. .
"VSports在线直播" Figures

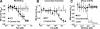
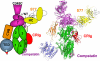
"VSports手机版" References
-
- Thurman, J. M., and Holers, V. M. (2006) J. Immunol. 176 1305–1310 - PubMed
-
- Janssen, B. J., Christodoulidou, A., McCarthy, A., Lambris, J. D., and Gros, P. (2006) Nature 444 213–216 - PubMed
-
- Janssen, B. J., Huizinga, E. G., Raaijmakers, H. C., Roos, A., Daha, M. R., Nilsson-Ekdahl, K., Nilsson, B., and Gros, P. (2005) Nature 437 505–511 - PubMed
Publication types
- VSports最新版本 - Actions
MeSH terms
- "VSports app下载" Actions
- "VSports在线直播" Actions
- Actions (VSports注册入口)
- "VSports手机版" Actions
- V体育2025版 - Actions
- "V体育官网" Actions
- "VSports" Actions
- Actions (VSports手机版)
- "VSports手机版" Actions
- Actions (V体育官网入口)
- V体育官网入口 - Actions
Substances
- V体育官网入口 - Actions
- VSports - Actions
- "V体育ios版" Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

