VSports最新版本 - The gene encoding early growth response 2, a target of the transcription factor NFAT, is required for the development and maturation of natural killer T cells
- PMID: 19169262
- PMCID: PMC2728767
- DOI: V体育ios版 - 10.1038/ni.1696
The gene encoding early growth response 2, a target of the transcription factor NFAT, is required for the development and maturation of natural killer T cells
Abstract
The influence of signals transmitted by the phosphatase calcineurin and the transcription factor NFAT on the development and function of natural killer T (NKT) cells is unclear. In this report, we demonstrate that the transcription factor early growth response 2 (Egr2), a target gene of NFAT, was specifically required for the ontogeny of NKT cells but not that of conventional CD4(+) or CD8(+) T cells VSports手机版. NKT cells developed normally in the absence of Egr1 or Egr3, which suggests that Egr2 is a specific regulator of NKT cell differentiation. We found that Egr2 was important in the selection, survival and maturation of NKT cells. Our findings emphasize the importance of the calcineurin-NFAT-Egr2 pathway in the development of the NKT lymphocyte lineage. .
VSports在线直播 - Conflict of interest statement
Competing Interests Statement: The authors declare competing financial interests: details accompany the full-text HTML version of the paper at http://www V体育安卓版. nature. com/natureimmunology/.
Figures
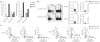
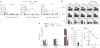
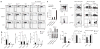
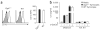
Comment in
-
Developing NKT cells need their calcium.Nat Immunol. 2009 Mar;10(3):231-3. doi: 10.1038/ni0309-231. Nat Immunol. 2009. PMID: 19221550 No abstract available.
References
-
- Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. - PubMed
-
- Vincent MS, Gumperz JE, Brenner MB. Understanding the function of CD1-restricted T cells. Nat Immunol. 2003;4:517–523. - PubMed
-
- Gapin L, Matsuda JL, Surh CD, Kronenberg M. NKT cells derive from double-positive thymocytes that are positively selected by CD1d. Nat Immunol. 2001;2:971–978. - PubMed
Publication types (VSports注册入口)
MeSH terms
- "V体育2025版" Actions
- "VSports app下载" Actions
- Actions (V体育平台登录)
- V体育2025版 - Actions
- V体育平台登录 - Actions
- "VSports app下载" Actions
Substances
- VSports - Actions
Grants and funding (V体育平台登录)
LinkOut - more resources
Full Text Sources (V体育平台登录)
Molecular Biology Databases
Research Materials (V体育2025版)

