The inhibitory action of kohamaic acid A derivatives on mammalian DNA polymerase beta
- PMID: 19127241
- PMCID: V体育2025版 - PMC6253949
- DOI: 10.3390/molecules14010102
The inhibitory action of kohamaic acid A derivatives on mammalian DNA polymerase beta
"V体育2025版" Abstract
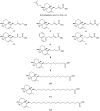
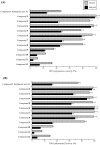
We previously isolated a novel natural product, designated kohamaic acid A (KA-A, compound 1), as an inhibitor of the first cleavage of fertilized sea urchin eggs, and found that this compound could selectively inhibit the activities of mammalian DNA polymerases (pols). In this paper, we investigated the structure and bioactivity of KA-A and its chemically synthesized 11 derivatives (i. e. , compounds 2-12), including KA-A - fatty acid conjugates. The pol inhibitory activity of compound 11 [(1S*,4aS*,8aS*)-17-(1,4,4a,5,6,7,8,8a-octahydro-2,5,5,8a-tetramethyl-naphthalen-1-yl)heptadecanoic acid] was the strongest among the synthesized compounds, and the range of IC(50) values for mammalian pols was 3. 22 to 8. 76 microM; therefore, the length of the fatty acid side chain group of KA-A is important for pol inhibition. KA-A derivatives could prevent human cancer cell (promyelocytic leukemia cell line, HL-60) growth with the same tendency as the inhibition of mammalian pols. Since pol beta is the smallest molecule, we used it to analyze the biochemical relationship with KA-A derivatives. From computer modeling analysis (i. e. , docking simulation analysis), these compounds bound selectively to four amino acid residues (Leu11, Lys35, His51 and Thr79) of the N-terminal 8-kDa domain of pol beta, and the binding energy between compound 11 and pol beta was largest in the synthesized compounds VSports手机版. The relationship between the three-dimensional molecular structures of KA-A-related compounds and these inhibitory activities is discussed. .
Figures


References
-
- Kokubo S., Yogi K., Uddin M. J., Inuzuka T., Suenaga K., Ueda K., Uemura D. Kohamaic acids A and B, novel cytotoxic sesterterpenic acids, from the marine sponge Ircinia sp. Chem. Lett. 2001;30:176–177.
-
- Mizushina Y., Murakami C., Yogi K., Ueda K., Ishidoh T., Takemura M., Perpelescu M., Suzuki M., Oshige M., Yamaguchi T., Saneyoshi M., Yoshida H., Sakaguchi K. Kohamaic acid A, a novel sesterterpenic acid, inhibits activities of DNA polymerases from deuterostomes. Biochim. Biophys. Acta. 2003;1648:55–61. - PubMed (V体育安卓版)
-
- Chang L. M. S. Phylogeny of DNA polymerase-β. Science. 1976;191:1183–1185. - PubMed
-
- Hobart P. M., Infante A. A. A low molecular weight DNA polymerase β in the sea urchin Strongylocentrotus purpurantus. Partial purification, properties, and changes in development. J. Biol. Chem. 1978;253:8229–8238. - PubMed
Publication types
MeSH terms
- Actions (VSports注册入口)
- VSports最新版本 - Actions
- V体育平台登录 - Actions
- Actions (V体育2025版)
- "V体育ios版" Actions
- VSports - Actions
- Actions (VSports手机版)
- VSports在线直播 - Actions
Substances
- "VSports app下载" Actions
- Actions (V体育安卓版)
LinkOut - more resources (VSports在线直播)
Full Text Sources (VSports注册入口)
Miscellaneous (V体育平台登录)

