Increasing Cu bioavailability inhibits Abeta oligomers and tau phosphorylation
- PMID: 19122148
- PMCID: PMC2626711
- DOI: V体育2025版 - 10.1073/pnas.0809057106
Increasing Cu bioavailability inhibits Abeta oligomers and tau phosphorylation (VSports注册入口)
Abstract
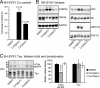
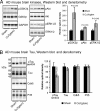
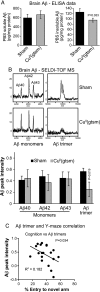
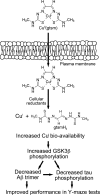
Cognitive decline in Alzheimer's disease (AD) involves pathological accumulation of synaptotoxic amyloid-beta (Abeta) oligomers and hyperphosphorylated tau. Because recent evidence indicates that glycogen synthase kinase 3beta (GSK3beta) activity regulates these neurotoxic pathways, we developed an AD therapeutic strategy to target GSK3beta. The strategy involves the use of copper-bis(thiosemicarbazonoto) complexes to increase intracellular copper bioavailability and inhibit GSK3beta through activation of an Akt signaling pathway. Our lead compound Cu(II)(gtsm) significantly inhibited GSK3beta in the brains of APP/PS1 transgenic AD model mice. Cu(II)(gtsm) also decreased the abundance of Abeta trimers and phosphorylated tau, and restored performance of AD mice in the Y-maze test to levels expected for cognitively normal animals VSports手机版. Improvement in the Y-maze correlated directly with decreased Abeta trimer levels. This study demonstrates that increasing intracellular copper bioavailability can restore cognitive function by inhibiting the accumulation of neurotoxic Abeta trimers and phosphorylated tau. .
Conflict of interest statement
The authors declare no conflict of interest.
Figures
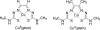
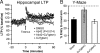
References
-
- Crouch PJ, et al. Mechanisms of Aβ mediated neurodegeneration in Alzheimer's disease. Int J Biochem Cell Biol. 2008;40:181–198. - PubMed
-
- Borchelt DR, et al. Familial Alzheimer's disease-linked presenilin 1 variants elevate Aβ1-42/1-40 ratio in vitro and in vivo. Neuron. 1996;17:1005–1013. - PubMed
-
- De Strooper B. Aph-1, Pen-2, and nicastrin with nresenilin generate an active γ-secretase complex. Neuron. 2003;38:9–12. - "V体育平台登录" PubMed
-
- Scheuner D, et al. Secreted amyloid β-protein similar to that in the senile plaques of Alzheimer's disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer's disease. Nat Med. 1996;2:864–870. - "VSports" PubMed
-
- Vassar R, et al. Beta-secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286:735–741. - PubMed
Publication types
MeSH terms
- "VSports注册入口" Actions
- Actions (V体育平台登录)
- Actions (VSports app下载)
- "V体育2025版" Actions
- V体育官网入口 - Actions
- "VSports手机版" Actions
- "VSports手机版" Actions
- "V体育官网入口" Actions
- "VSports app下载" Actions
- VSports手机版 - Actions
Substances
- Actions (VSports)
- "VSports在线直播" Actions
- "VSports app下载" Actions
LinkOut - more resources (V体育平台登录)
Full Text Sources (VSports在线直播)
Medical
Molecular Biology Databases
Miscellaneous

