Retinoic acid receptor signaling levels and antigen dose regulate gut homing receptor expression on CD8+ T cells (V体育2025版)
- PMID: 19079159
- PMCID: PMC6485487
- DOI: 10.1038/mi.2007.4
"V体育ios版" Retinoic acid receptor signaling levels and antigen dose regulate gut homing receptor expression on CD8+ T cells
Abstract (VSports最新版本)
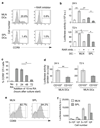
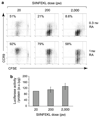
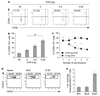
Recent studies have highlighted a central role for intestinal dendritic cells (DCs) and vitamin A metabolite retinoic acid (RA) in the generation of alpha4beta7(+) CCR9(+)"gut tropic" effector T cells VSports手机版. Here, using RA-responsive element reporter mice, we demonstrate that both splenic and mesenteric lymph node (MLN) DCs enhanced retinoic acid receptor (RAR) signaling in CD8(+) T cells; however, only a subset of MLN DCs, expressing the integrin alpha-chain CD103, induced an early RAR signal that is required for efficient CCR9 induction. MLN-primed CD8(+) T cells also received enhanced RAR-dependent signals compared with splenic-primed CD8(+) T cells in vivo. Further DC-mediated induction of gut homing receptors was inhibited at a high antigen dose without influencing RAR signaling events, and resulted in less efficient CD8(+) T-cell entry into the small intestinal mucosa. These results highlight a complex interplay between antigen dose and DC subset-induced RAR signaling events in the generation of tissue tropic effector T-cell subsets. .
Conflict of interest statement
The authors declare no conflict of interest.
Figures

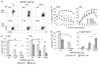
References (VSports注册入口)
-
- Kunkel EJ, Campbell JJ, Haraldsen G, Pan J, Boisvert J, Roberts AI, et al. Lymphocyte CC chemokine receptor 9 and epithelial thymus-expressed chemokine (TECK) expression distinguish the small intestinal immune compartment: epithelial expression of tissue-specific chemokines as an organizing principle in regional immunity. J Exp Med. 2000;192:761–768. - PMC - PubMed
-
- Svensson M, Marsal J, Ericsson A, Carramolino L, Broden T, Marquez G, et al. CCL25 mediates the localization of recently activated CD8alphabeta(+) lymphocytes to the small-intestinal mucosa. J Clin Invest. 2002;110:1113–1121. - "VSports在线直播" PMC - PubMed
-
- Zabel BA, Agace WW, Campbell JJ, Heath HM, Parent D, Robert AI, et al. Human G protein-coupled receptor GPR-96/CC chemokine receptor 9 is selectively expressed on intestinal homing T lymphocytes, mucosal lymphocytes, and thymocytes and is required for thymus-expressed chemokine-mediated chemotaxis. J Exp Med. 1999;190:1241–1256. - PMC - PubMed
-
- Wurbel MA, Philippe JM, Nguyen C, Victorero G, Freeman T, Wooding P, et al. The chemokine TECK is expressed by thymic and intestinal epithelial cells and attracts double- and single-positive thymocytes expressing the TECK receptor CCR9. Eur J Immunol. 2000;30:262–271. - PubMed
V体育官网 - Publication types
- VSports在线直播 - Actions
MeSH terms
- VSports - Actions
- Actions (V体育官网入口)
- Actions (V体育ios版)
- "V体育官网" Actions
- "VSports在线直播" Actions
- "VSports注册入口" Actions
- Actions (V体育官网)
- "VSports app下载" Actions
Substances
- V体育2025版 - Actions
- V体育官网入口 - Actions
- "VSports app下载" Actions
Grants and funding
"VSports" LinkOut - more resources
Full Text Sources
Research Materials

