"V体育官网入口" Critical role of ROR-γt in a new thymic pathway leading to IL-17-producing invariant NKT cell differentiation
- PMID: 19057011
- PMCID: PMC2604995 (VSports最新版本)
- DOI: 10.1073/pnas.0806472105
V体育ios版 - Critical role of ROR-γt in a new thymic pathway leading to IL-17-producing invariant NKT cell differentiation
Abstract
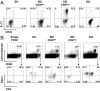
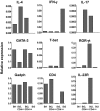
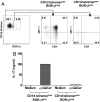
Invariant natural killer T (iNKT) cells constitute a subpopulation of T cells that recognize glycolipids presented by CD1d molecules. They are characterized by their prompt production of interleukin-4 (IL-4) and interferon-gamma (IFN-gamma), which enables them to modulate diverse immune responses. Recently, we enlarged this concept by identifying a distinct IL-17-producing iNKT cell subset, named iNKT17 cells. The mechanisms leading to the acquisition of this new iNKT cell activity are unknown. Herein we show that IL-17-producing iNKT cells are already present in the thymus, predominantly among a subset regarded so far as an immature stage of thymic iNKT cell development, the CD1d tetramer(pos)CD44(pos)NK1. 1(neg)CD4(neg) cells. Using EGFP reporter mice, we demonstrate that the transcription factor ROR-gammat is critical for the thymic differentiation of this subset because only ROR-gammat(pos) iNKT cells are capable of massively secreting IL-17. Moreover, IL-17-producing CD1d tetramer(pos)CD44(pos)NK1. 1(neg)CD4(neg) thymic iNKT cells have reached a mature differentiation stage because they fail to generate other cell subsets in fetal thymic organ culture. Conversely, thymic ROR-gammat(neg) iNKT cell precursors give rise to progeny, but acquire neither ROR-gammat expression nor the ability to secrete IL-17. In conclusion, our findings demonstrate an alternative thymic pathway leading to the development of iNKT17 cells that requires ROR-gammat expression. VSports手机版.
"VSports手机版" Conflict of interest statement
The authors declare no conflict of interest.
Figures





"V体育官网" References
-
- Mosmann TR, Coffman RL. TH1 and TH2 Cells: Different Patterns of Lymphokine Secretion Lead to Different Functional Properties. Annu Rev Immunol. 1989;7:145–173. - "VSports app下载" PubMed
-
- Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol. 2002;2:933–944. - PubMed
-
- Langrish CL, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. - PMC (VSports注册入口) - PubMed
-
- Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: An effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–688. - PubMed
-
- Kawaguchi M, Adachi M, Oda N, Kokubu F, Huang S-K. IL-17 cytokine family. J Allergy Clin Immunol. 2004;114:1265–1273. - PubMed
Publication types
MeSH terms
- Actions (V体育ios版)
- "V体育2025版" Actions
- "VSports最新版本" Actions
- VSports手机版 - Actions
- Actions (VSports最新版本)
- Actions (V体育平台登录)
Substances
- Actions (VSports注册入口)
- V体育安卓版 - Actions
- VSports在线直播 - Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

