IL-17A enhances vitamin D3-induced expression of cathelicidin antimicrobial peptide in human keratinocytes
- PMID: 19050268
- PMCID: PMC2655307
- DOI: 10.4049/jimmunol.181.12.8504 (V体育官网)
IL-17A enhances vitamin D3-induced expression of cathelicidin antimicrobial peptide in human keratinocytes
Abstract
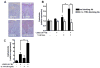
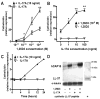
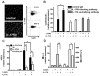
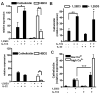
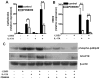
Cathelicidin is strongly expressed in lesional skin in psoriasis and may play an important role as both an antimicrobial peptide and as an autoinflammatory mediator in this chronic skin disease. The mechanism of increased cathelicidin in psoriatic keratinocytes is not known, but recent observations have found that psoriasis has abundant Th17 cells that produce IL-17A and IL-22. We found that human keratinocytes stimulated with supernatants from T cells isolated from lesional psoriatic skin increased expression of cathelicidin when stimulated in the presence of 1,25-dihydroxyvitamin D(3) (1,25D(3)). This increase was signaled through the IL-17RA. In vitro, IL-17A, but not IL-22, enhanced cathelicidin mRNA and peptide expression in keratinocytes dependent on the presence of 1,25D(3). At the same time, coincubation with 1,25D(3) blocked induction of human beta-defensin 2 (HBD2), IL-6, and IL-8, which are other target genes of IL-17A VSports手机版. Act1, an adaptor associated with IL-17RA and essential for IL-17A signaling, mediated cathelicidin induction, as its suppression by small interfering RNA inhibited HBD2 and cathelicidin. Both, 1,25D(3) and IL-17A signaled cathelicidin induction through MEK-ERK. These results suggest that increased IL-17A in psoriatic skin increases cathelicidin through a vitamin D(3)-, Act1-, and MEK-ERK-dependent mechanism. Therapy targeting this cathelicidin-regulating system might be beneficial in patients suffering from psoriasis. .
Figures (VSports手机版)

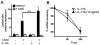
References
-
- Nickoloff BJ, Qin JZ, Nestle FO. Immunopathogenesis of psoriasis. Clin Rev Allergy Immunol. 2007;33:45–56. - PubMed (V体育2025版)
-
- Lande R, Gregorio J, Facchinetti V, Chatterjee B, Wang YH, Homey B, Cao W, Wang YH, Su B, Nestle FO, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564–569. - "V体育官网入口" PubMed
-
- Agerberth B, Charo J, Werr J, Olsson B, Idali F, Lindbom L, Kiessling R, Jornvall H, Wigzell H, Gudmundsson GH. The human antimicrobial and chemotactic peptides LL-37 and α- defensins are expressed by specific lymphocyte and monocyte populations. Blood. 2000;96:3086–3093. - PubMed
Publication types
MeSH terms
- "VSports在线直播" Actions
- "VSports注册入口" Actions
- "VSports注册入口" Actions
- VSports注册入口 - Actions
- "V体育ios版" Actions
- "VSports" Actions
- Actions (VSports)
- V体育安卓版 - Actions
- "V体育平台登录" Actions
- "V体育官网" Actions
Substances
- "V体育平台登录" Actions
- Actions (V体育官网)
- "V体育官网" Actions
- V体育安卓版 - Actions
- VSports - Actions
Grants and funding
"VSports" LinkOut - more resources
V体育官网 - Full Text Sources
Other Literature Sources
Miscellaneous

