Cholangiocytes as immune modulators in rotavirus-induced murine biliary atresia
- PMID: 19040538
- PMCID: PMC3783657
- DOI: "V体育平台登录" 10.1111/j.1478-3231.2008.01921.x
"VSports最新版本" Cholangiocytes as immune modulators in rotavirus-induced murine biliary atresia
"VSports在线直播" Abstract
Background/aims: Biliary atresia (BA) is a progressive disease characterized by bile duct inflammation and fibrosis. The aetiology is unknown and may be due to a virus-induced, autoimmune-mediated injury of cholangiocytes VSports手机版. Cholangiocytes are not only targets of injury but may also modulate hepatic inflammation. The aim of this study was to determine the immune profile of murine cholangiocytes and the ability to function as antigen-presenting cells (APCs) in culture with Rhesus rotavirus (RRV), poly I:C (viral mimic) or interferon-gamma/tumour necrosis factor-alpha. .
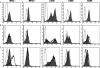
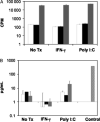
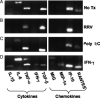
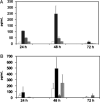
Methods/results: Both the cholangiocyte cell line (long-term culture) and fresh, ex vivo cholangiocytes expressed APC surface markers major histocompatibility complex (MHC)-class I and II and CD40, while only the cultured cell line expressed costimulatory molecules B7-1 and B7-2. Despite APC expression, cultured cholangiocytes were unable to function as competent APCs in T-cell proliferation assays. Furthermore, both cultured and ex vivo cholangiocytes expressed RNA transcripts for many pro-inflammatory cytokines and chemokines. V体育安卓版.
Conclusions: Although cholangiocytes contain APC molecules, they are incompetent at antigen presentation and cannot elicit effective T-cell activation. Upregulation of MHC-class I and II found in BA mice may serve to prime the cholangiocyte as a target for immune-mediated injury V体育ios版. Cholangiocytes produced many pro-inflammatory cytokines and chemokines in the setting of RRV infection and T-helper type 1 cytokine milieu, suggesting a role of cholangiocytes as immune modulators promoting the ongoing inflammation that exists in RRV-induced BA. .
VSports app下载 - Figures



References
-
- Chardot C, Carton M, Spire-Bendelac N, et al. Epidemiology of biliary atresia in France: a national study 1986–96. J Hepatol. 1999;31:1006–13. - "VSports" PubMed
-
- Chen SM, Chang MH, the Taiwan Infant Stool Color Card Study Group et al. Screening for biliary atresia by infant stool color card in Taiwan. Pediatrics. 2006;117:1147–54. - PubMed
-
- Karrer FM, Price MR, Bensard DD, et al. Long-term results with the Kasai operation for biliary atresia. Arch Surg. 1996;131:493–6. - PubMed
-
- Karrer FM, Bensard DD. Neonatal cholestasis. Semin Pediatr Surg. 2000;9:166–9. - PubMed
-
- Mack CL, Sokol RJ. Unraveling the pathogenesis and etiology of biliary atresia. Pediatr Res. 2005;57:87R–94R. - PubMed
Publication types
MeSH terms
- "V体育官网入口" Actions
- "VSports最新版本" Actions
- "VSports注册入口" Actions
- V体育2025版 - Actions
- "V体育官网" Actions
- Actions (V体育安卓版)
- V体育2025版 - Actions
- Actions (VSports)
Substances
- "VSports最新版本" Actions
Grants and funding
LinkOut - more resources
V体育官网入口 - Full Text Sources
Other Literature Sources
"V体育ios版" Medical
Research Materials
Miscellaneous

