Glucose metabolism inhibits apoptosis in neurons and cancer cells by redox inactivation of cytochrome c
- PMID: 19029908
- PMCID: VSports - PMC2626347
- DOI: 10.1038/ncb1807 (VSports手机版)
V体育2025版 - Glucose metabolism inhibits apoptosis in neurons and cancer cells by redox inactivation of cytochrome c
Abstract
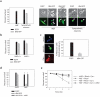
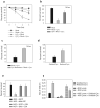
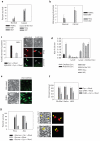
Neurons and cancer cells use glucose extensively, yet the precise advantage of this adaptation remains unclear. These two seemingly disparate cell types also show an increased regulation of the apoptotic pathway, which allows for their long-term survival. Here we show that both neurons and cancer cells strictly inhibit cytochrome c-mediated apoptosis by a mechanism dependent on glucose metabolism. We report that the pro-apoptotic activity of cytochrome c is influenced by its redox state and that increases in reactive oxygen species (ROS) following an apoptotic insult lead to the oxidation and activation of cytochrome c VSports手机版. In healthy neurons and cancer cells, however, cytochrome c is reduced and held inactive by intracellular glutathione (GSH), generated as a result of glucose metabolism by the pentose phosphate pathway. These results uncover a striking similarity in apoptosis regulation between neurons and cancer cells and provide insight into an adaptive advantage offered by the Warburg effect for cancer cell evasion of apoptosis and for long-term neuronal survival. .
Figures

References (VSports app下载)
-
- Wright KM, Deshmukh M. Restricting apoptosis for postmitotic cell survival and its relevance to cancer. Cell Cycle. 2006;5:1616–1620. - PubMed
-
- Yuan J, Yankner BA. Apoptosis in the nervous system. Nature. 2000;407:802–809. - PubMed
-
- Green DR, Evan GI. A matter of life and death. Cancer Cell. 2002;1:19–30. - PubMed
-
- Wang X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001;15:2922–2933. - PubMed
-
- Schubert D. Glucose metabolism and Alzheimer's disease. Ageing Res Rev. 2005;4:240–257. - PubMed
Publication types
- V体育2025版 - Actions
- Actions (VSports在线直播)
MeSH terms
- Actions (V体育ios版)
- "V体育官网" Actions
- Actions (V体育官网入口)
- "V体育官网入口" Actions
- V体育2025版 - Actions
- "VSports app下载" Actions
- VSports注册入口 - Actions
- "V体育2025版" Actions
- "VSports在线直播" Actions
Substances
- Actions (V体育官网)
Grants and funding
LinkOut - more resources
Full Text Sources

