Farnesoid X receptor antagonizes nuclear factor kappaB in hepatic inflammatory response
- PMID: 18972444
- PMCID: V体育2025版 - PMC3056574
- DOI: 10.1002/hep.22519
Farnesoid X receptor antagonizes nuclear factor kappaB in hepatic inflammatory response (V体育2025版)
"V体育2025版" Abstract
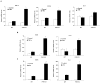
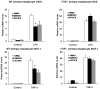
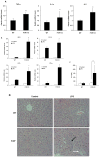
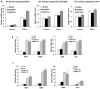
The farnesoid X receptor (FXR) is a nuclear receptor that plays key roles in hepatoprotection by maintaining the homeostasis of liver metabolism. FXR null mice display strong hepatic inflammation and develop spontaneous liver tumors. In this report, we demonstrate that FXR is a negative modulator of nuclear factor kappaB (NF-kappaB)-mediated hepatic inflammation. Activation of FXR by its agonist ligands inhibited the expression of inflammatory mediators in response to NF-kappaB activation in both HepG2 cells and primary hepatocytes cultured in vitro VSports手机版. In vivo, compared with wild-type controls, FXR(-/-) mice displayed elevated messenger RNA (mRNA) levels of inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), interferon-inducible protein 10, and interferon-gamma in response to lipopolysaccharide (LPS). Examination of FXR(-/-) livers showed massive necroses and inflammation after treatment with LPS at a dose that does not induce significant liver damage or inflammation in wild-type mice. Moreover, transfection of a constitutively active FXR expression construct repressed the iNOS, COX-2, interferon-inducible protein 10 and interferon-gamma mRNA levels induced by LPS administration. FXR activation had no negative effects on NF-kappaB-activated antiapoptotic genes, suggesting that FXR selectively inhibits the NF-kappaB-mediated hepatic inflammatory response but maintains or even enhances the cell survival response. On the other hand, NF-kappaB activation suppressed FXR-mediated gene expression both in vitro and in vivo, indicating a negative crosstalk between the FXR and NF-kappaB signaling pathways. Our findings reveal that FXR is a negative mediator of hepatic inflammation, which may contribute to the critical roles of FXR in hepatoprotection and suppression of hepatocarcinogenesis. .
Conflict of interest statement (VSports app下载)
Figures
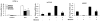
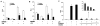

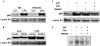
Comment in
-
Targeting farnesoid x receptor in hepatic and biliary inflammatory diseases.Gastroenterology. 2009 Aug;137(2):734-5; discussion 736. doi: 10.1053/j.gastro.2009.06.027. Epub 2009 Jun 27. Gastroenterology. 2009. PMID: 19563842 No abstract available.
References
-
- Gujral JS, Farhood A, Bajt ML, Jaeschke H. Neutrophils aggravate acute liver injury during obstructive cholestasis in bile duct-ligated mice. Hepatology. 2003;38:355–363. - PubMed
-
- Gu X, Ke S, Liu D, Sheng T, Thomas PE, Rabson AB, Gallo MA, et al. Role of NF-kappaB in regulation of PXR-mediated gene expression: a mechanism for the suppression of cytochrome P-450 3A4 by proinflammatory agents. J Biol Chem. 2006;281:17882–17889. - PubMed
-
- Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–759. - PubMed
-
- Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich-Pyest E, et al. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–466. - PubMed
-
- Elsharkawy AM, Mann DA. Nuclear factor-kappaB and the hepatic inflammation-fibrosis-cancer axis. Hepatology. 2007;46:590–597. - PubMed (VSports手机版)
V体育平台登录 - Publication types
- "VSports app下载" Actions
MeSH terms
- Actions (VSports)
- VSports在线直播 - Actions
- V体育官网入口 - Actions
- "V体育2025版" Actions
- VSports在线直播 - Actions
- Actions (VSports在线直播)
- Actions (V体育官网入口)
- "V体育官网入口" Actions
- Actions (V体育官网)
- V体育官网 - Actions
- V体育官网入口 - Actions
- Actions (V体育官网入口)
- Actions (V体育ios版)
- Actions (VSports)
Substances
- Actions (VSports注册入口)
- Actions (VSports app下载)
- VSports app下载 - Actions
- VSports app下载 - Actions
- "VSports app下载" Actions
Grants and funding
"VSports app下载" LinkOut - more resources
"V体育官网入口" Full Text Sources
Other Literature Sources (VSports在线直播)
Molecular Biology Databases
Research Materials
