The immunity-related GTPase Irgm1 promotes the expansion of activated CD4+ T cell populations by preventing interferon-gamma-induced cell death (V体育ios版)
- PMID: 18806793
- PMCID: PMC2580721
- DOI: 10.1038/ni.1653
The immunity-related GTPase Irgm1 promotes the expansion of activated CD4+ T cell populations by preventing interferon-gamma-induced cell death (V体育2025版)
"VSports app下载" Abstract
Mice deficient in the interferon-gamma (IFN-gamma)-inducible, immunity-related GTPase Irgm1 have defective host resistance to a variety of intracellular pathogens. This greater susceptibility to infection is associated with impaired IFN-gamma-dependent macrophage microbicidal activity in vitro. Here we show that Irgm1 also regulated the survival of mature effector CD4(+) T lymphocytes by protecting them from IFN-gamma-induced autophagic cell death VSports手机版. Mice deficient in both IFN-gamma and Irgm1 were 'rescued' from the lymphocyte depletion and greater mortality that occurs in mice singly deficient in Irgm1 after mycobacterial infection. Our studies identify a feedback mechanism in the T helper type 1 response that limits the detrimental effects of IFN-gamma on effector T lymphocyte survival while promoting the antimicrobial functions of IFN-gamma. .
Conflict of interest statement
The authors have no conflicting financial interests.
Figures
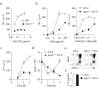


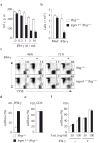
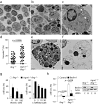
Comment in
-
IFN-gamma and self-absorbed CD4+ T cells: a regulatory double negative.Nat Immunol. 2008 Nov;9(11):1210-2. doi: 10.1038/ni1108-1210. Nat Immunol. 2008. PMID: 18936780 Free PMC article.
References
-
- Gett AV, Sallusto F, Lanzavecchia A, Geginat J. T cell fitness determined by signal strength. Nat Immunol. 2003;4:355–60. - PubMed
-
- Croft M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat Rev Immunol. 2003;3:609–20. - VSports - PubMed
-
- Krammer PH, Arnold R, Lavrik IN. Life and death in peripheral T cells. Nat Rev Immunol. 2007;7:532–42. - PubMed
-
- Green DR. Fas Bim boom! Immunity. 2008;28:141–3. - PubMed
Publication types (V体育官网入口)
MeSH terms
- V体育2025版 - Actions
- VSports手机版 - Actions
- VSports最新版本 - Actions
- "V体育平台登录" Actions
- VSports手机版 - Actions
- Actions (VSports在线直播)
- "VSports在线直播" Actions
- "VSports" Actions
- "VSports在线直播" Actions
- VSports注册入口 - Actions
- Actions (V体育官网)
- Actions (VSports最新版本)
- "VSports在线直播" Actions
- "V体育2025版" Actions
- "VSports手机版" Actions
Substances
- "V体育ios版" Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
VSports app下载 - Research Materials

